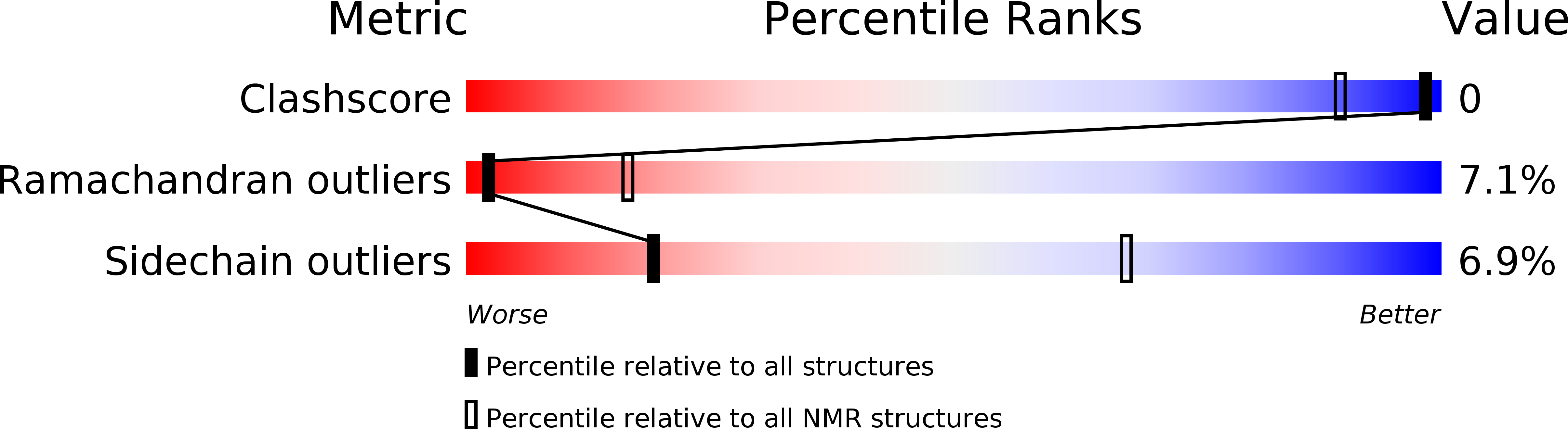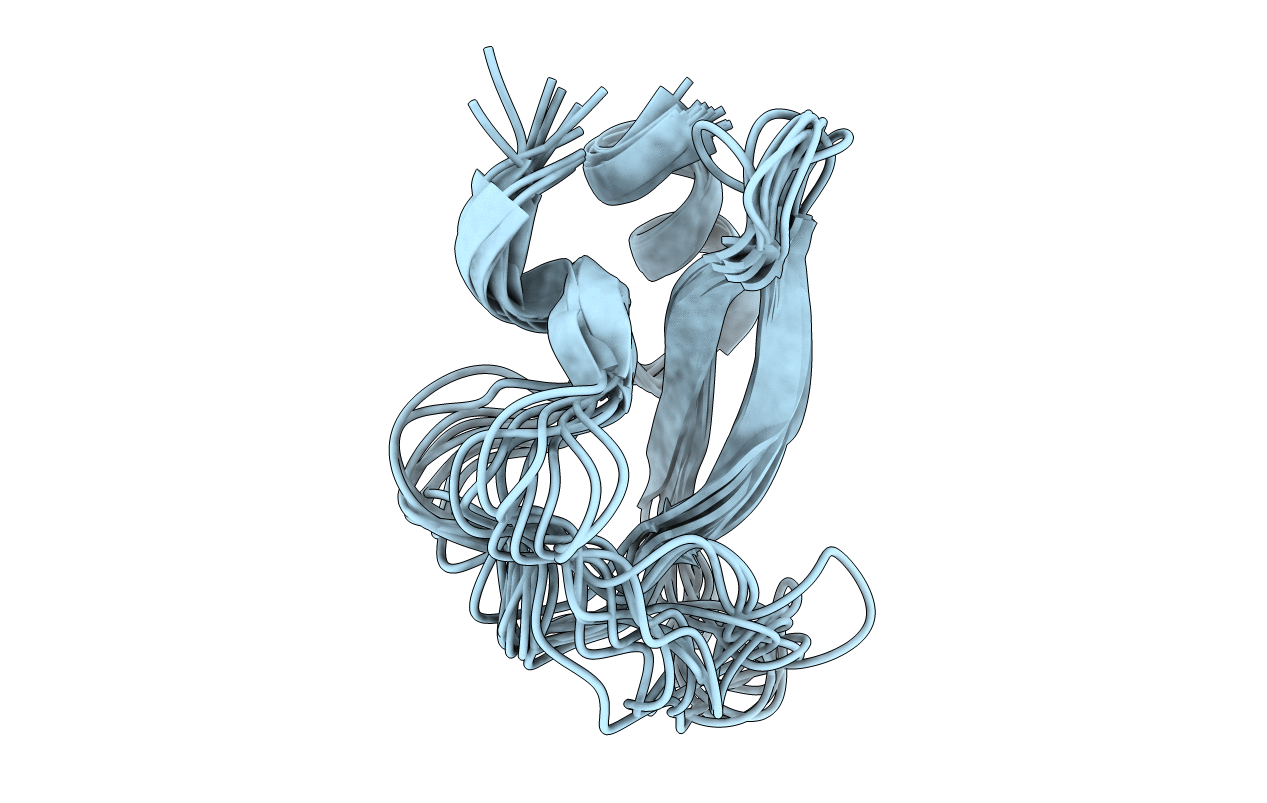
Deposition Date
1994-10-31
Release Date
1995-10-31
Last Version Date
2024-11-20
Entry Detail
PDB ID:
1TCP
Keywords:
Title:
NMR STRUCTURE DETERMINATION OF TICK ANTICOAGULANT PEPTIDE (TAP)
Biological Source:
Source Organism(s):
Ornithodoros moubata (Taxon ID: 6938)
Expression System(s):
Method Details:
Experimental Method:
Conformers Submitted:
10