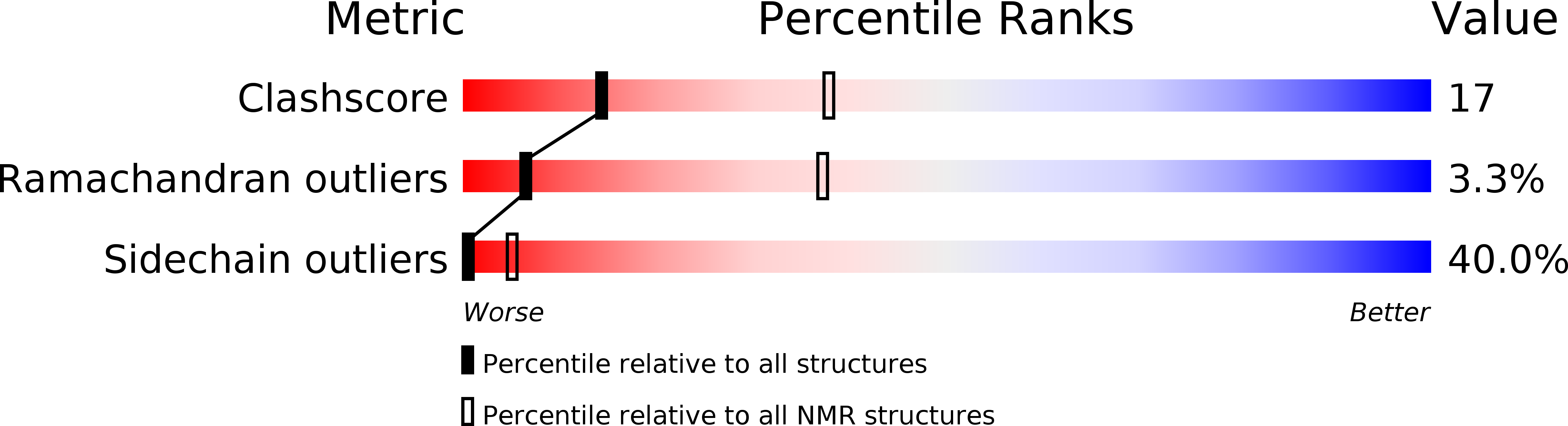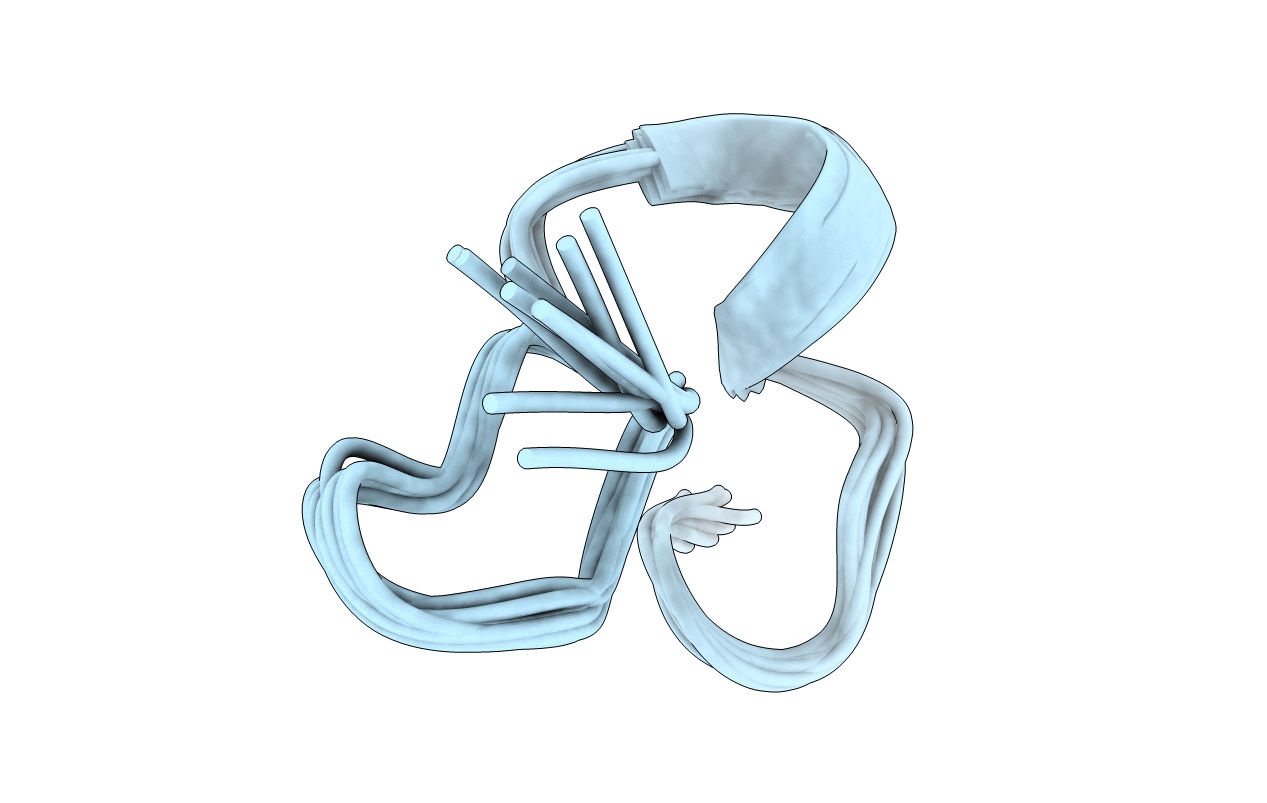
Deposition Date
1992-12-12
Release Date
1994-01-31
Last Version Date
2025-03-26
Entry Detail
PDB ID:
1TCJ
Keywords:
Title:
STRUCTURE-ACTIVITY RELATIONSHIPS OF MU-CONOTOXIN GIIIA: STRUCTURE DETERMINATION OF ACTIVE AND INACTIVE SODIUM CHANNEL BLOCKER PEPTIDES BY NMR AND SIMULATED ANNEALING CALCULATIONS
Biological Source:
Source Organism(s):
Conus geographus (Taxon ID: 6491)
Method Details:
Experimental Method:
Conformers Submitted:
10