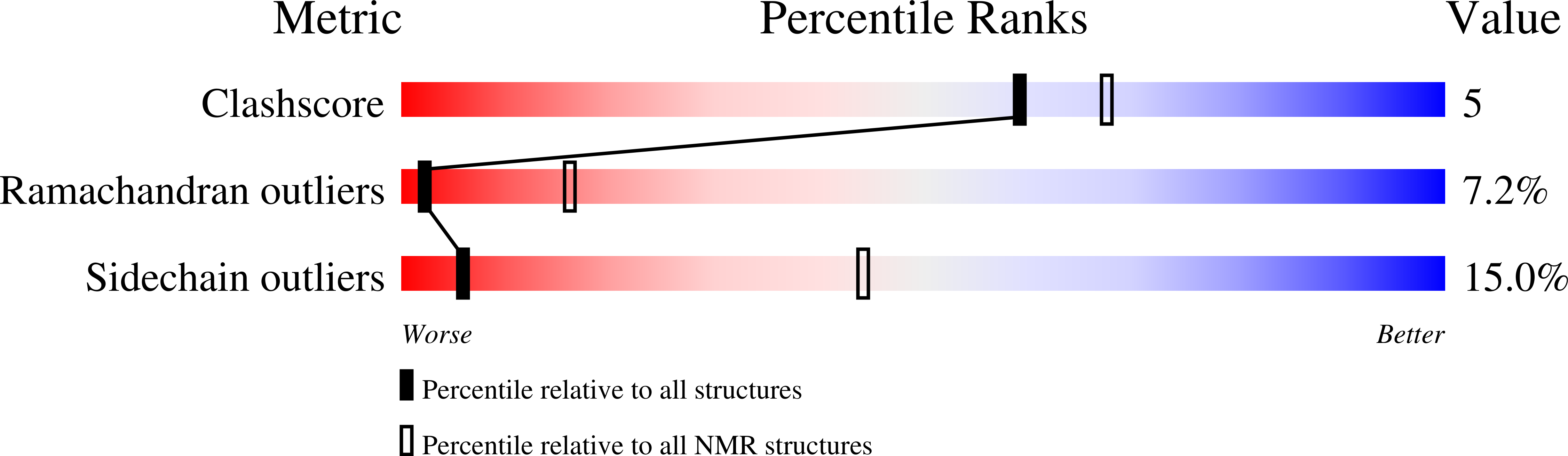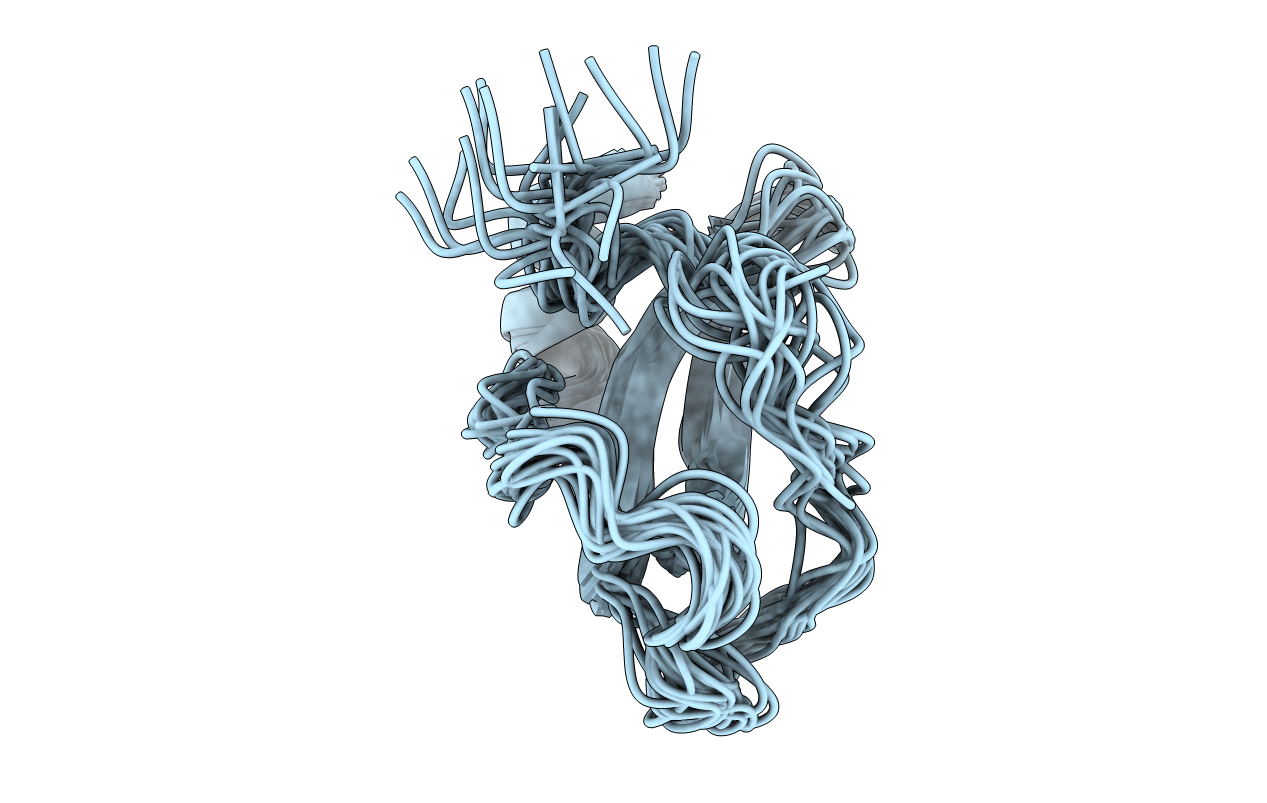
Deposition Date
1994-08-16
Release Date
1994-11-30
Last Version Date
2024-11-20
Entry Detail
PDB ID:
1TAP
Keywords:
Title:
NMR SOLUTION STRUCTURE OF RECOMBINANT TICK ANTICOAGULANT PROTEIN (RTAP), A FACTOR XA INHIBITOR FROM THE TICK ORNITHODOROS MOUBATA
Biological Source:
Source Organism(s):
Ornithodoros moubata (Taxon ID: 6938)
Method Details:
Experimental Method:
Conformers Submitted:
20