Deposition Date
2004-05-06
Release Date
2004-08-10
Last Version Date
2024-02-14
Entry Detail
PDB ID:
1T6L
Keywords:
Title:
Crystal Structure of the Human Cytomegalovirus DNA Polymerase Subunit, UL44
Biological Source:
Source Organism(s):
Human herpesvirus 5 (Taxon ID: 10359)
Expression System(s):
Method Details:
Experimental Method:
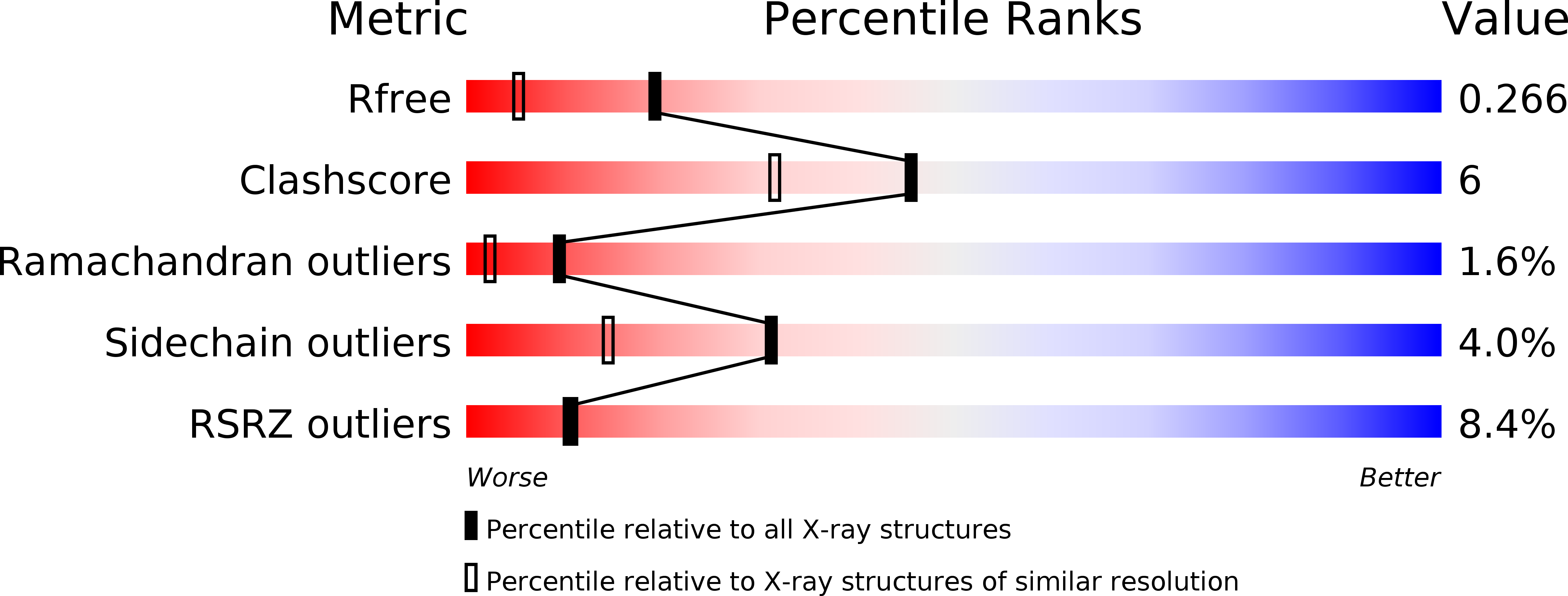
Resolution:
1.85 Å
R-Value Free:
0.26
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 61 2 2