Deposition Date
1992-03-12
Release Date
1992-10-15
Last Version Date
2024-02-14
Entry Detail
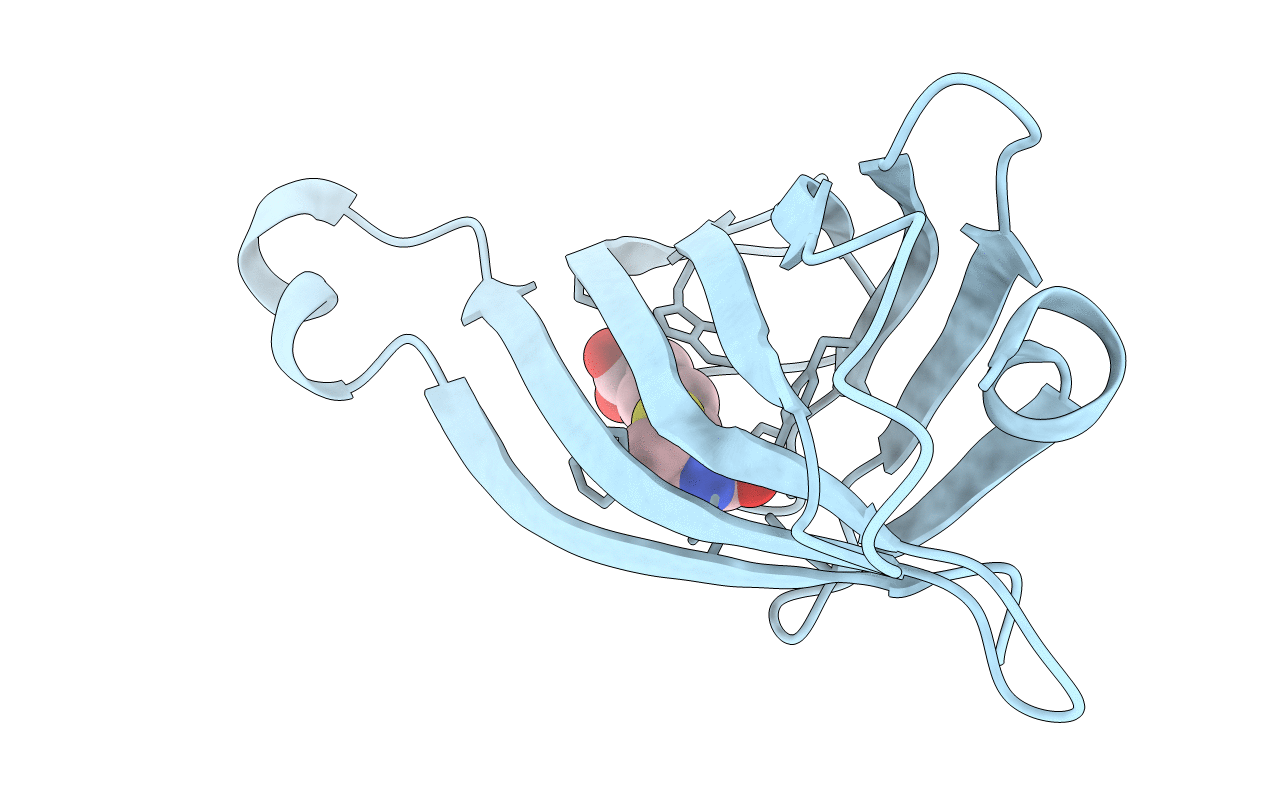
PDB ID:
1STP
Keywords:
Title:
STRUCTURAL ORIGINS OF HIGH-AFFINITY BIOTIN BINDING TO STREPTAVIDIN
Biological Source:
Source Organism(s):
Streptomyces avidinii (Taxon ID: 1895)
Method Details:
Experimental Method:
Resolution:
2.60 Å
R-Value Observed:
0.22
Space Group:
I 41 2 2


