Deposition Date
2004-03-17
Release Date
2004-04-06
Last Version Date
2024-11-20
Entry Detail
PDB ID:
1SQ6
Keywords:
Title:
Plasmodium falciparum homolog of Uridine phosphorylase/Purine nucleoside phosphorylase
Biological Source:
Source Organism:
Plasmodium falciparum (Taxon ID: 36329)
Host Organism:
Method Details:
Experimental Method:
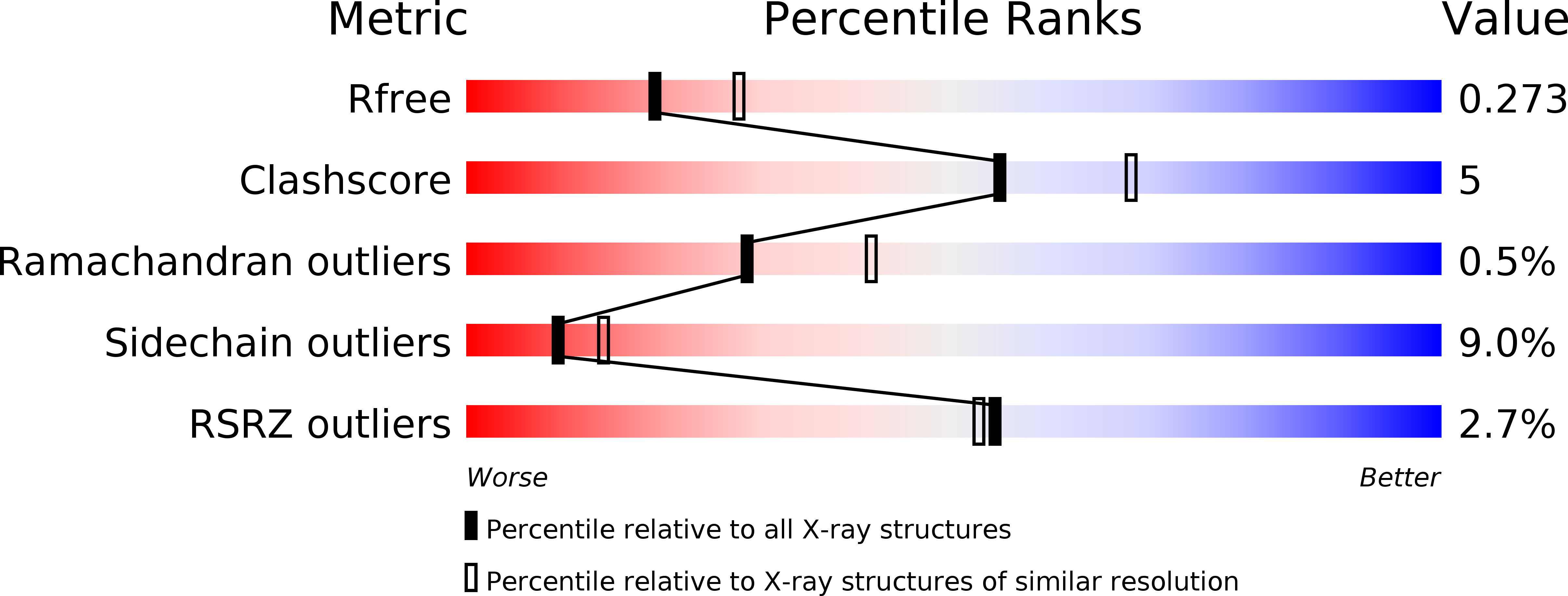
Resolution:
2.40 Å
R-Value Free:
0.26
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
H 3 2