Deposition Date
1992-02-11
Release Date
1993-04-15
Last Version Date
2024-11-06
Entry Detail
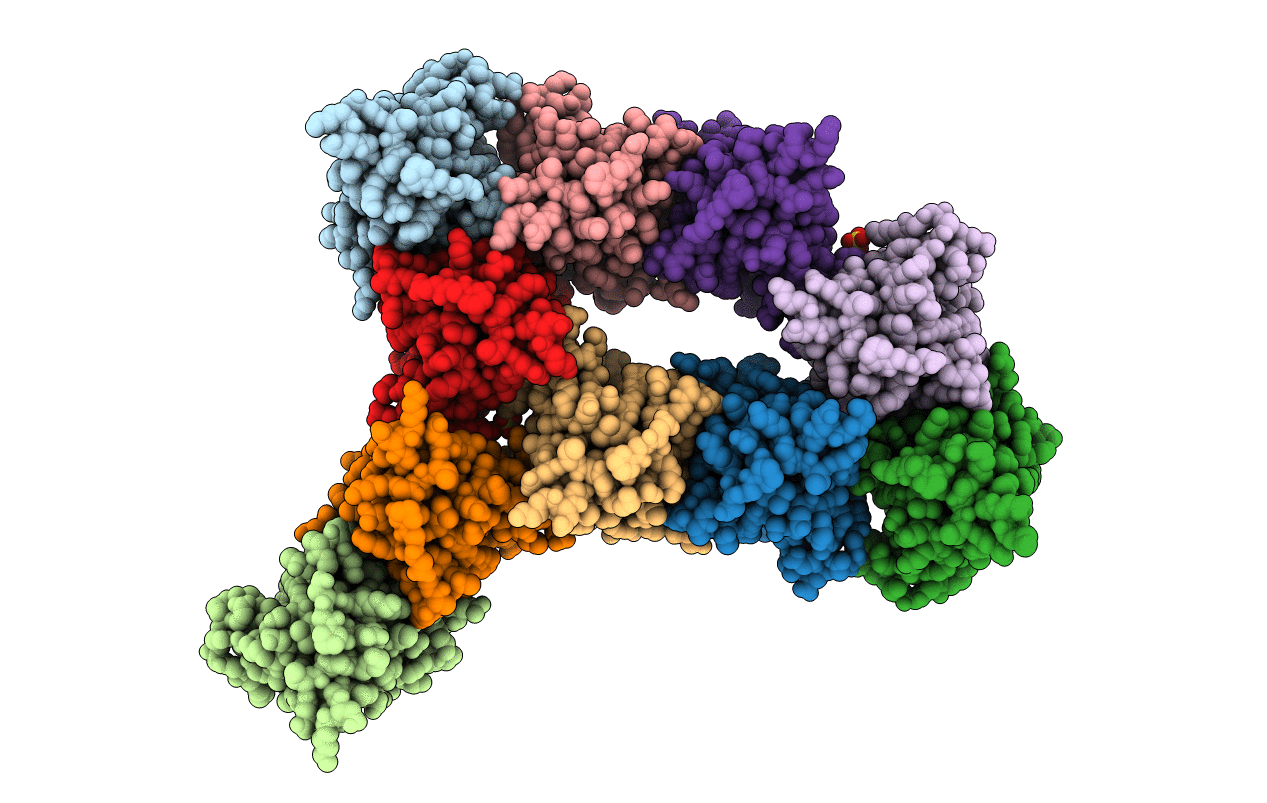
PDB ID:
1SOS
Keywords:
Title:
ATOMIC STRUCTURES OF WILD-TYPE AND THERMOSTABLE MUTANT RECOMBINANT HUMAN CU, ZN SUPEROXIDE DISMUTASE
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Method Details:


