Deposition Date
2004-03-08
Release Date
2004-08-03
Last Version Date
2024-07-10
Entry Detail
PDB ID:
1SM1
Keywords:
Title:
COMPLEX OF THE LARGE RIBOSOMAL SUBUNIT FROM DEINOCOCCUS RADIODURANS WITH QUINUPRISTIN AND DALFOPRISTIN
Biological Source:
Source Organism(s):
STREPTOMYCES GRAMINOFACIEN (Taxon ID: 68212)
DEINOCOCCUS RADIODURANS (Taxon ID: 1299)
DEINOCOCCUS RADIODURANS (Taxon ID: 1299)
Method Details:
Experimental Method:
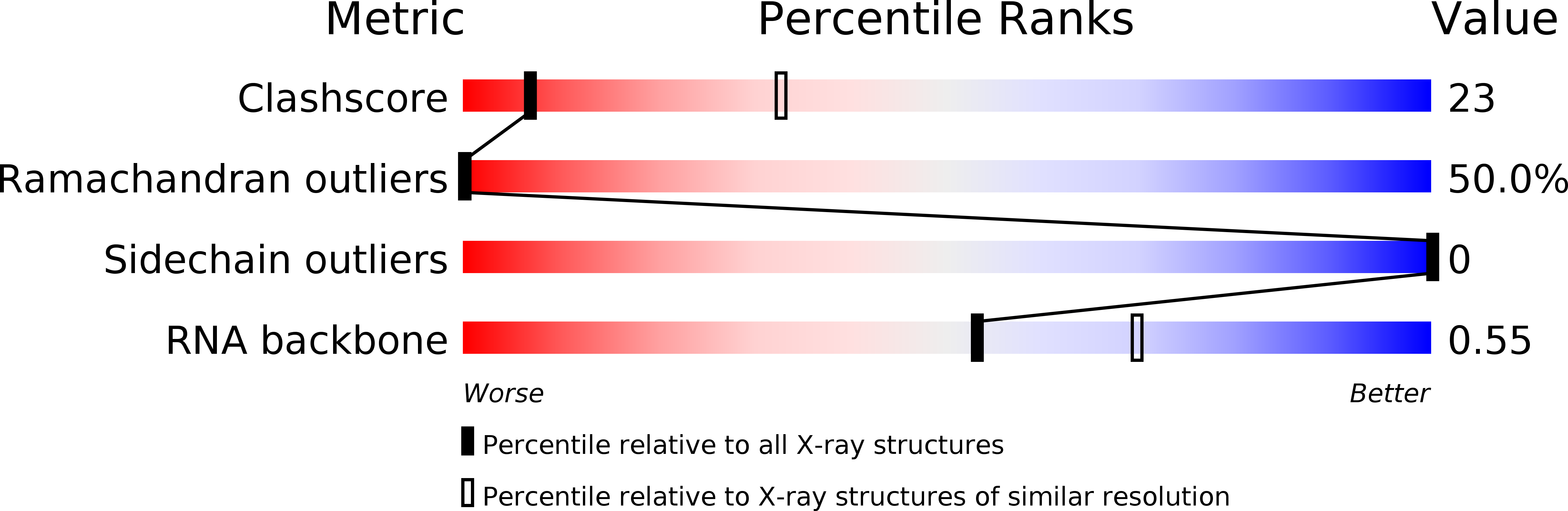
Resolution:
3.42 Å
R-Value Free:
0.34
R-Value Work:
0.27
R-Value Observed:
0.27
Space Group:
I 2 2 2