Abstact
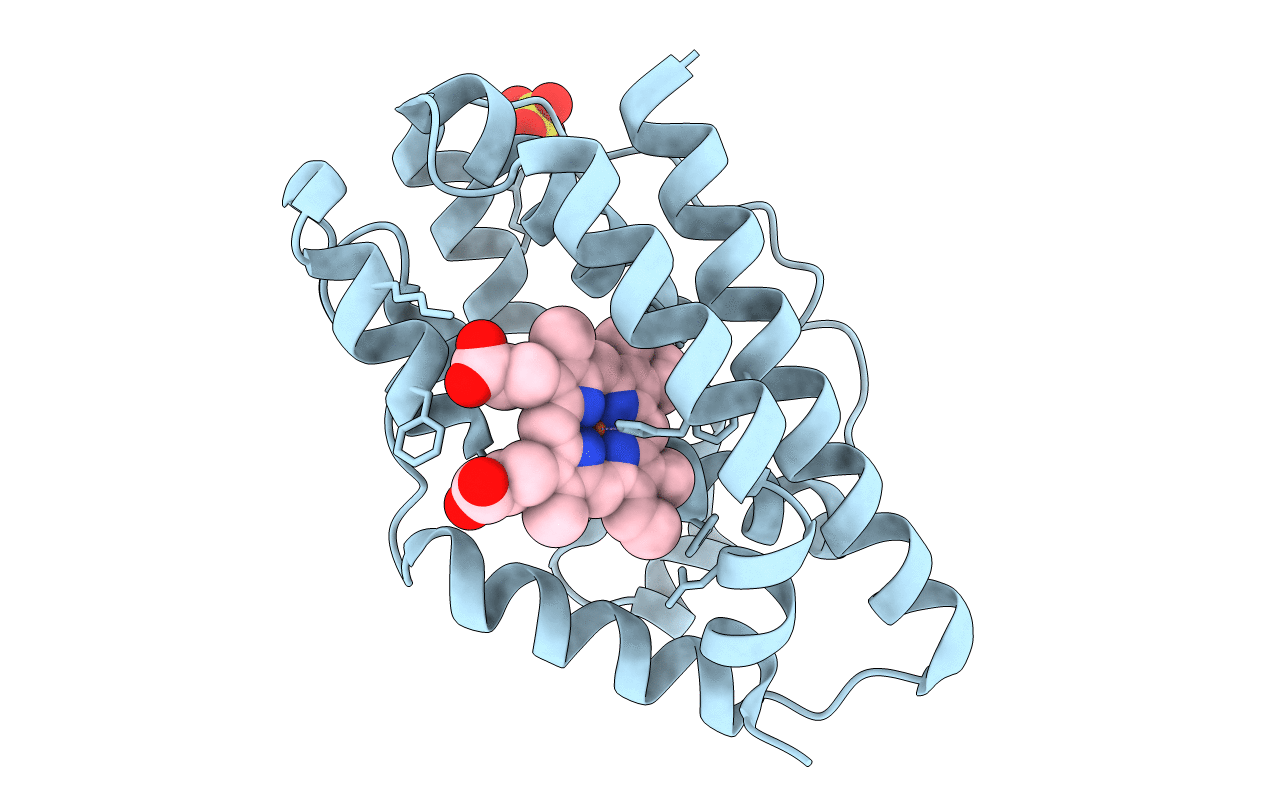
The Gram-negative bacterium Pseudomonas aeruginosa contains a heme oxygenase (pa-HO) that primarily oxygenates the delta-meso heme carbon [Caignan, G. A., Deshmukh, R., Wilks, A., Zeng, Y., Huang, H. W., Moenne-Loccoz, P., Bunce, R. A., Eastman, M. A., and Rivera, M. (2002) J. Am. Chem. Soc. 124, 14879-14892]. This differs from other previously characterized heme oxygenases, which display regioselectivity for the alpha-meso heme carbon. Here we report the crystal structure of pa-HO at 1.60 A resolution and compare it to the 1.50 A structure of nm-HO from Neisseria meningitidis [Schuller, D. J., Zhu, W., Stojiljkovic, I., Wilks, A., and Poulos, T. L. (2001) Biochemistry 40, 11552-11558]. The crystal structure of pa-HO maintains the same overall fold as other bacterial and mammalian heme oxygenases, including a conserved network of hydrogen-bonded solvent molecules important for dioxygen activation. The novel delta-regioselectivity of heme oxygenation observed by pa-HO is due to the heme being rotated by approximately 100 degrees, which places the delta-meso heme carbon in the same position as the alpha-meso heme carbon in other heme oxygenases. The main interaction in pa-HO that stabilizes the unique heme orientation is a salt bridge between Lys132 and the heme 7-propionate, as well as hydrophobic contacts involving Leu29, Val33, and Phe189 with the heme methyl and vinyl groups.