Deposition Date
1992-11-17
Release Date
1994-01-31
Last Version Date
2024-11-06
Entry Detail
PDB ID:
1SHP
Keywords:
Title:
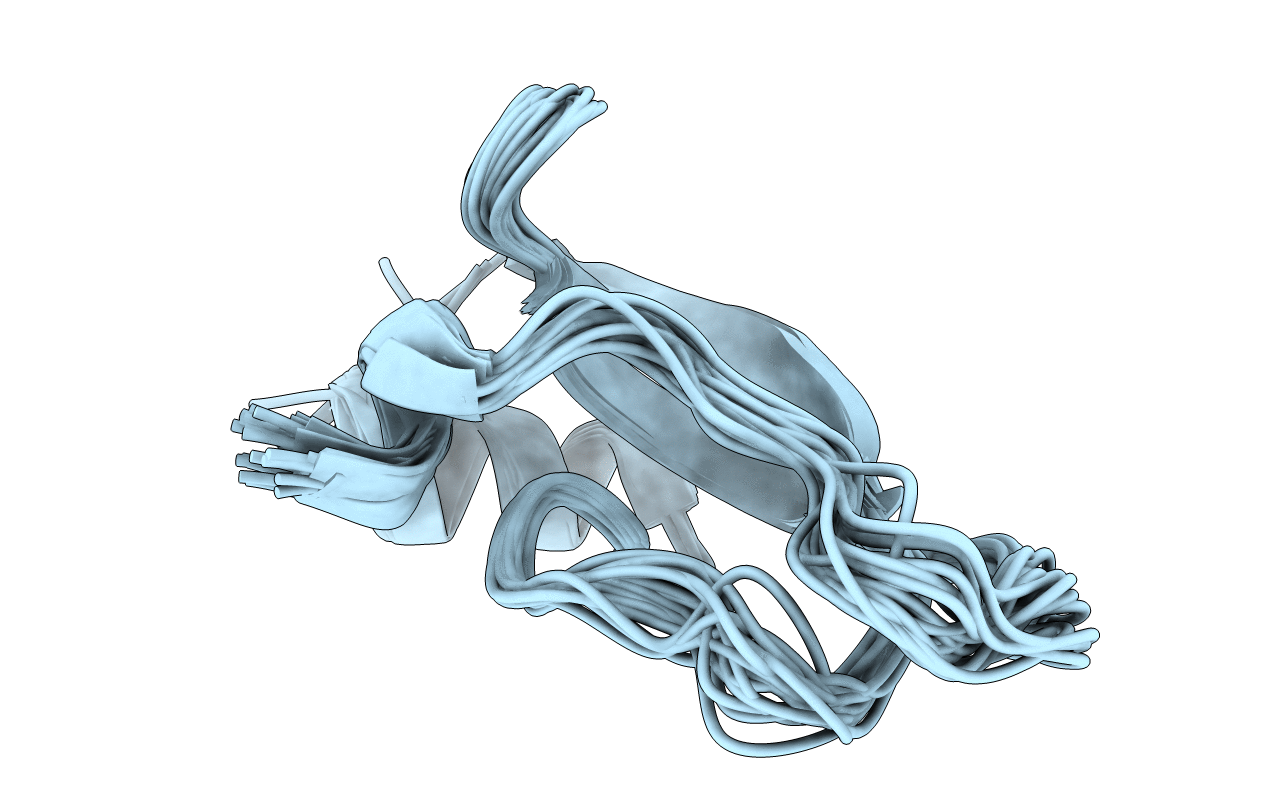
THE NMR SOLUTION STRUCTURE OF A KUNITZ-TYPE PROTEINASE INHIBITOR FROM THE SEA ANEMONE STICHODACTYLA HELIANTHUS
Biological Source:
Source Organism(s):
Stichodactyla helianthus (Taxon ID: 6123)
Method Details:
Experimental Method:
Conformers Submitted:
20