Deposition Date
1990-05-03
Release Date
1991-10-15
Last Version Date
2024-10-16
Entry Detail
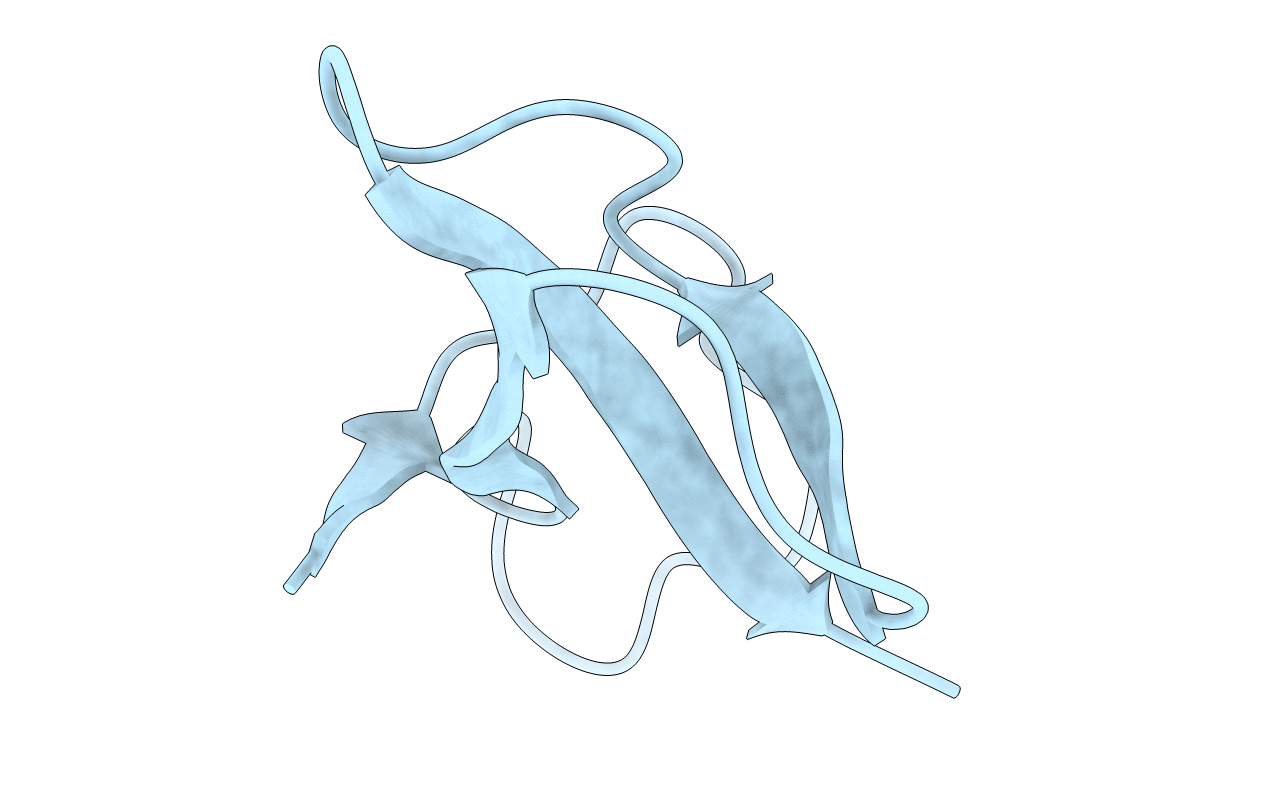
PDB ID:
1SH1
Keywords:
Title:
SOLUTION STRUCTURE OF NEUROTOXIN I FROM THE SEA ANEMONE STICHODACTYLA HELIANTHUS. A NUCLEAR MAGNETIC RESONANCE, DISTANCE GEOMETRY AND RESTRAINED MOLECULAR DYNAMICS STUDY
Biological Source:
Source Organism(s):
Stichodactyla helianthus (Taxon ID: 6123)
Method Details:
Experimental Method:
Conformers Submitted:
1


