Deposition Date
2004-02-24
Release Date
2004-06-22
Last Version Date
2024-11-13
Entry Detail
PDB ID:
1SGL
Keywords:
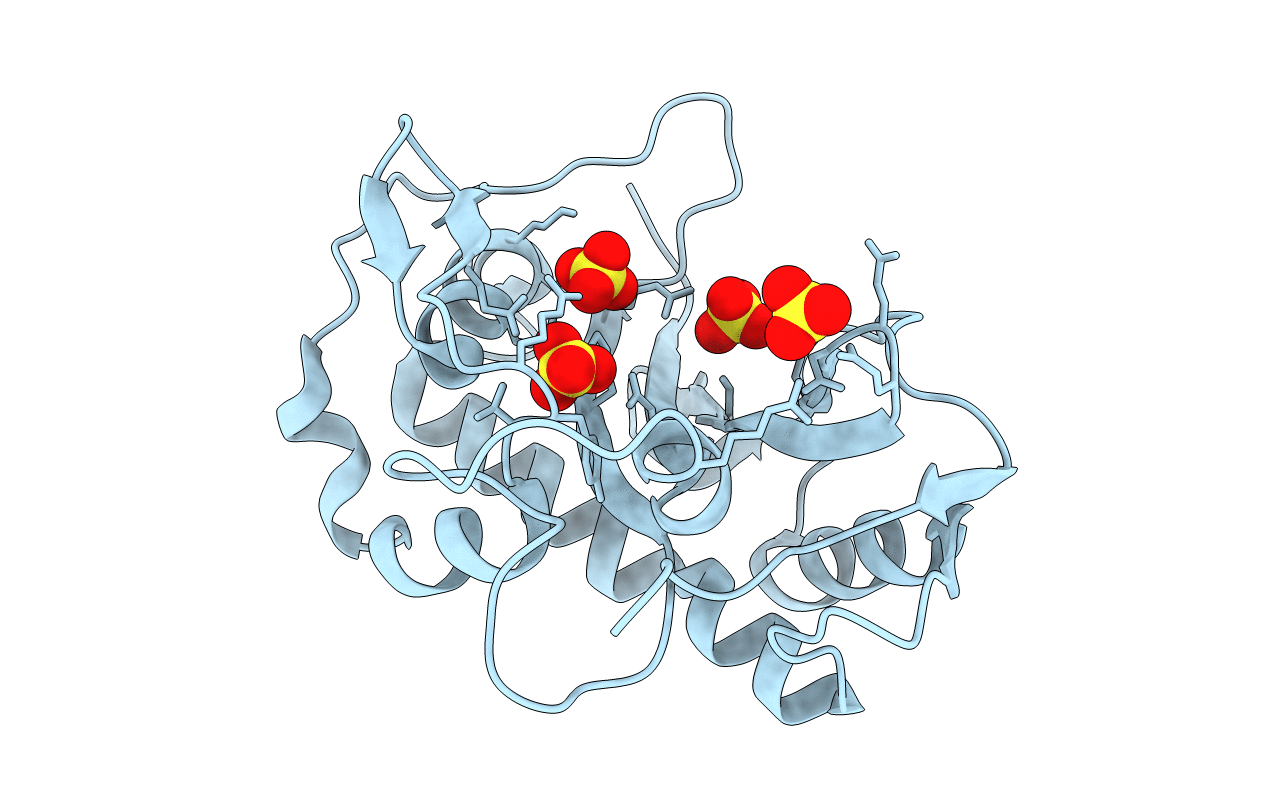
Title:
The three-dimensional structure and X-ray sequence reveal that trichomaglin is a novel S-like ribonuclease
Biological Source:
Source Organism(s):
Trichosanthes lepiniana (Taxon ID: 282652)
Method Details:
Experimental Method:
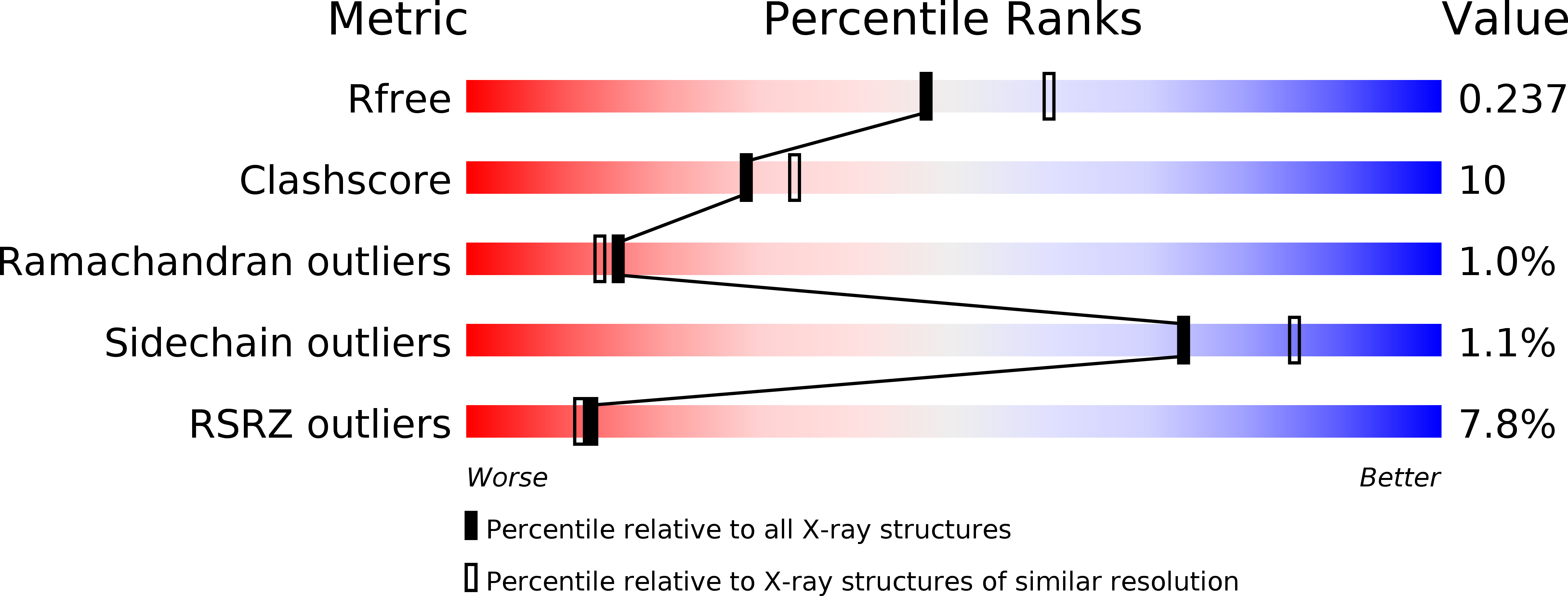
Resolution:
2.20 Å
R-Value Free:
0.23
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 61