Deposition Date
1993-07-19
Release Date
1993-10-31
Last Version Date
2024-02-14
Entry Detail
PDB ID:
1SBP
Keywords:
Title:
1.7 ANGSTROMS REFINED STRUCTURE OF SULFATE-BINDING PROTEIN INVOLVED IN ACTIVE TRANSPORT AND NOVEL MODE OF SULFATE BINDING
Biological Source:
Source Organism(s):
Salmonella typhimurium (Taxon ID: 602)
Method Details:
Experimental Method:
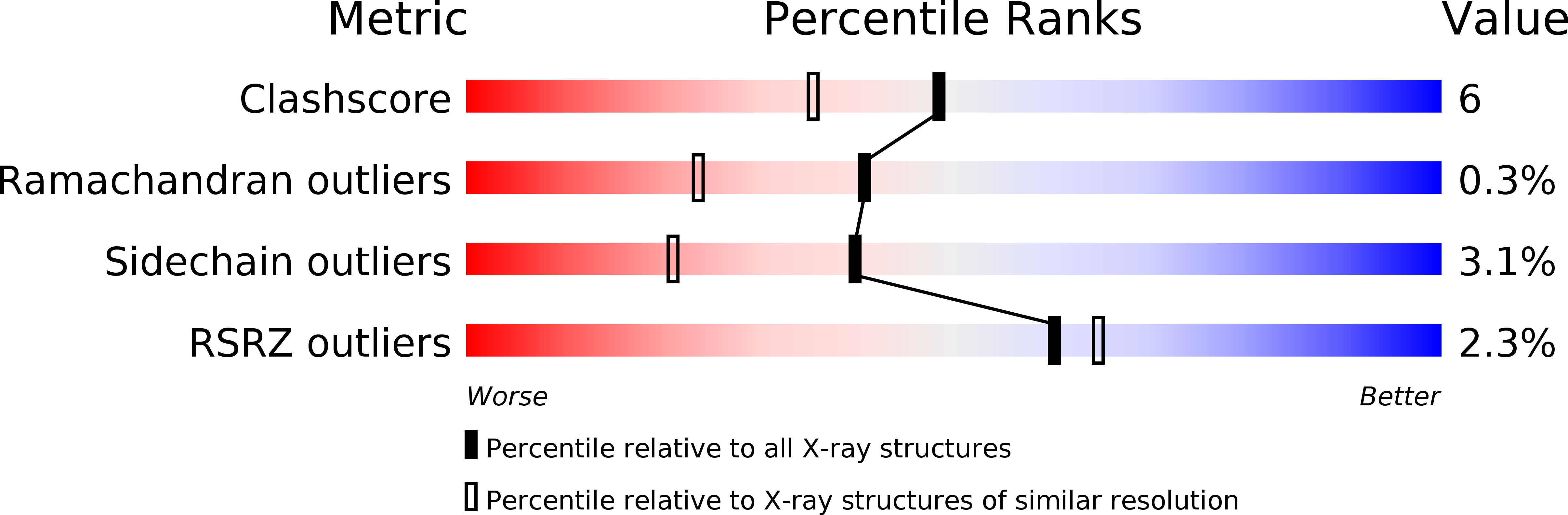
Resolution:
1.70 Å
R-Value Observed:
0.17
Space Group:
P 21 21 21