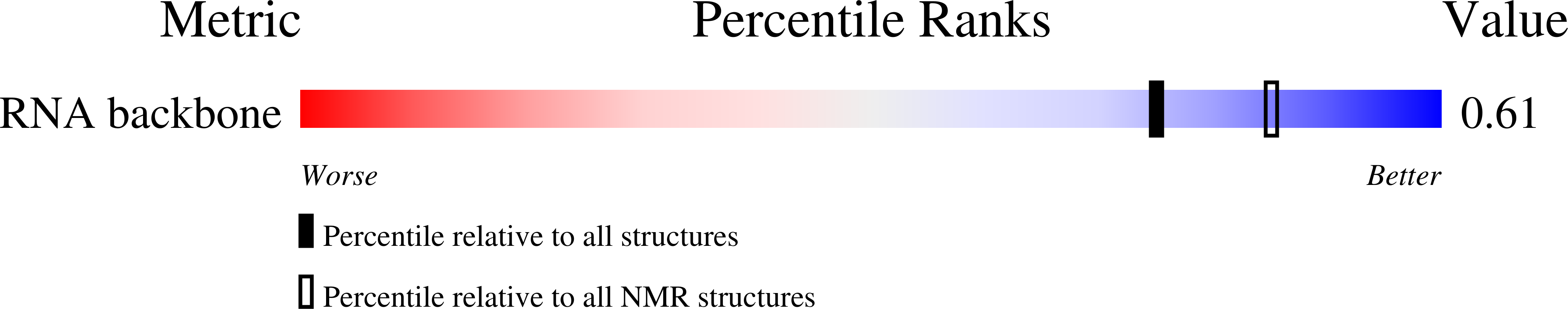
Deposition Date
2004-01-08
Release Date
2004-08-31
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1S2F
Keywords:
Title:
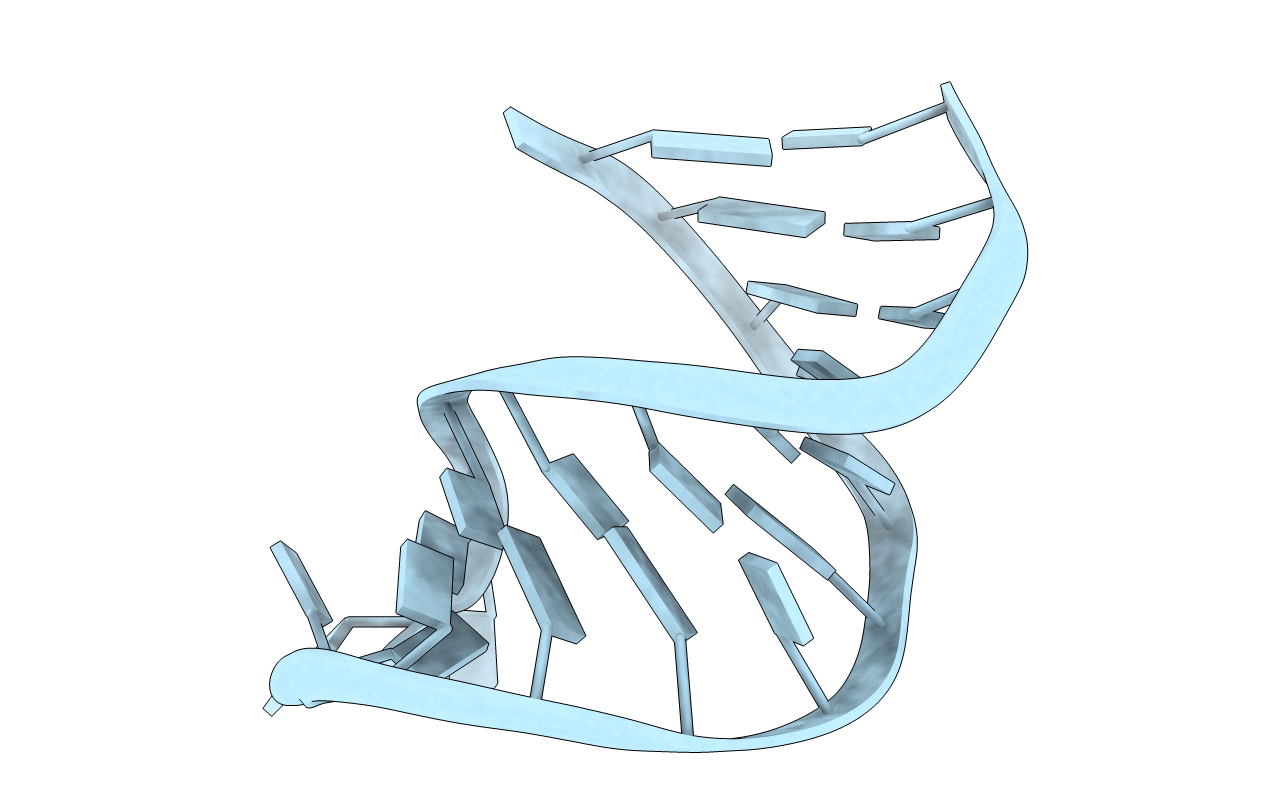
Average solution structure of a pseudo-5'-splice site from the negative regulator of splicing of Rous Sarcoma virus
Method Details:
Experimental Method:
Conformers Submitted:
1