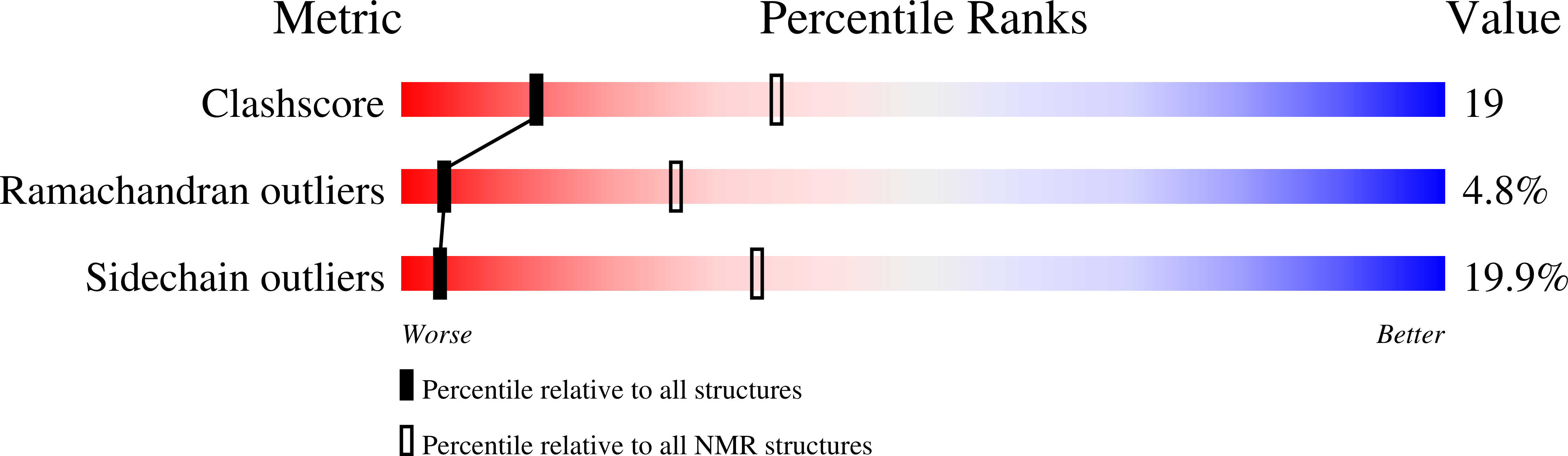
Deposition Date
2004-01-08
Release Date
2004-05-04
Last Version Date
2024-05-22
Entry Detail
Biological Source:
Source Organism(s):
Pseudomonas oleovorans (Taxon ID: 301)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
20
Conformers Submitted:
20
Selection Criteria:
The submitted conformer models are the 20 structures with the lowest