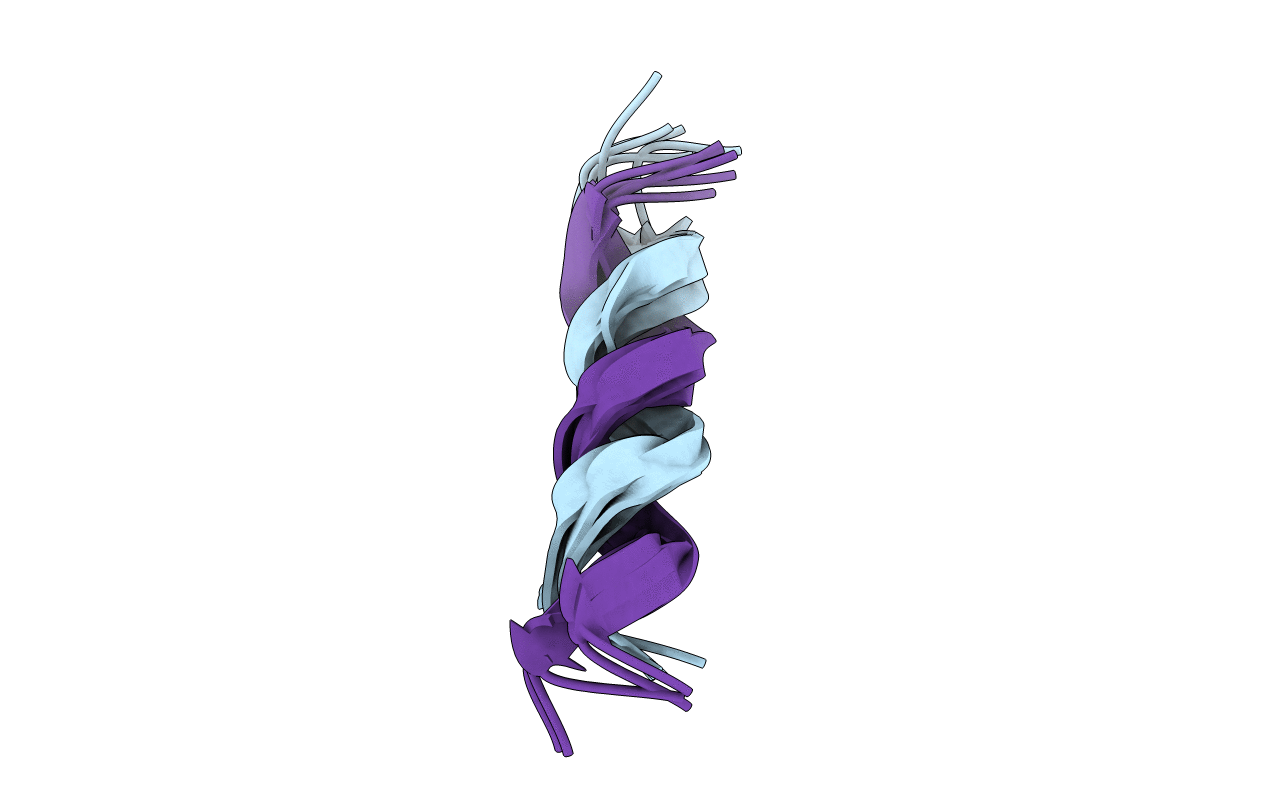
Deposition Date
2004-01-07
Release Date
2004-02-24
Last Version Date
2023-11-15
Entry Detail
PDB ID:
1S1O
Keywords:
Title:
NMR Structure of a D,L Alternating pentadecamer of norleucine: double antiparallel beta-helix
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
5
Selection Criteria:
structures with the lowest energy