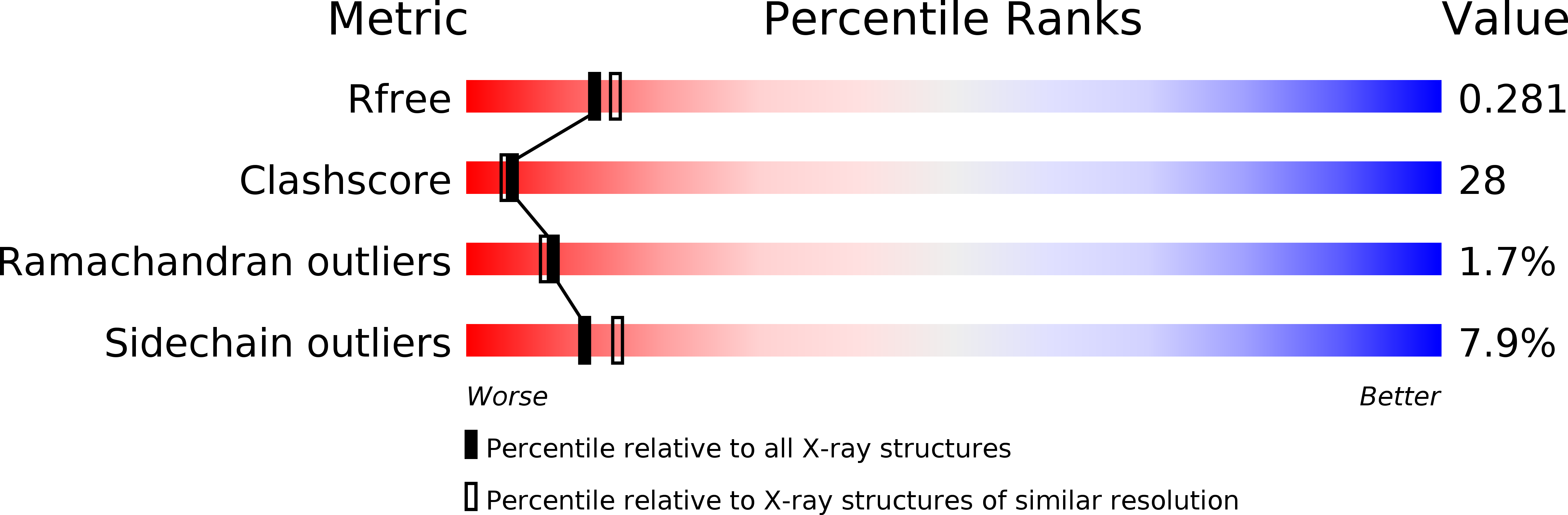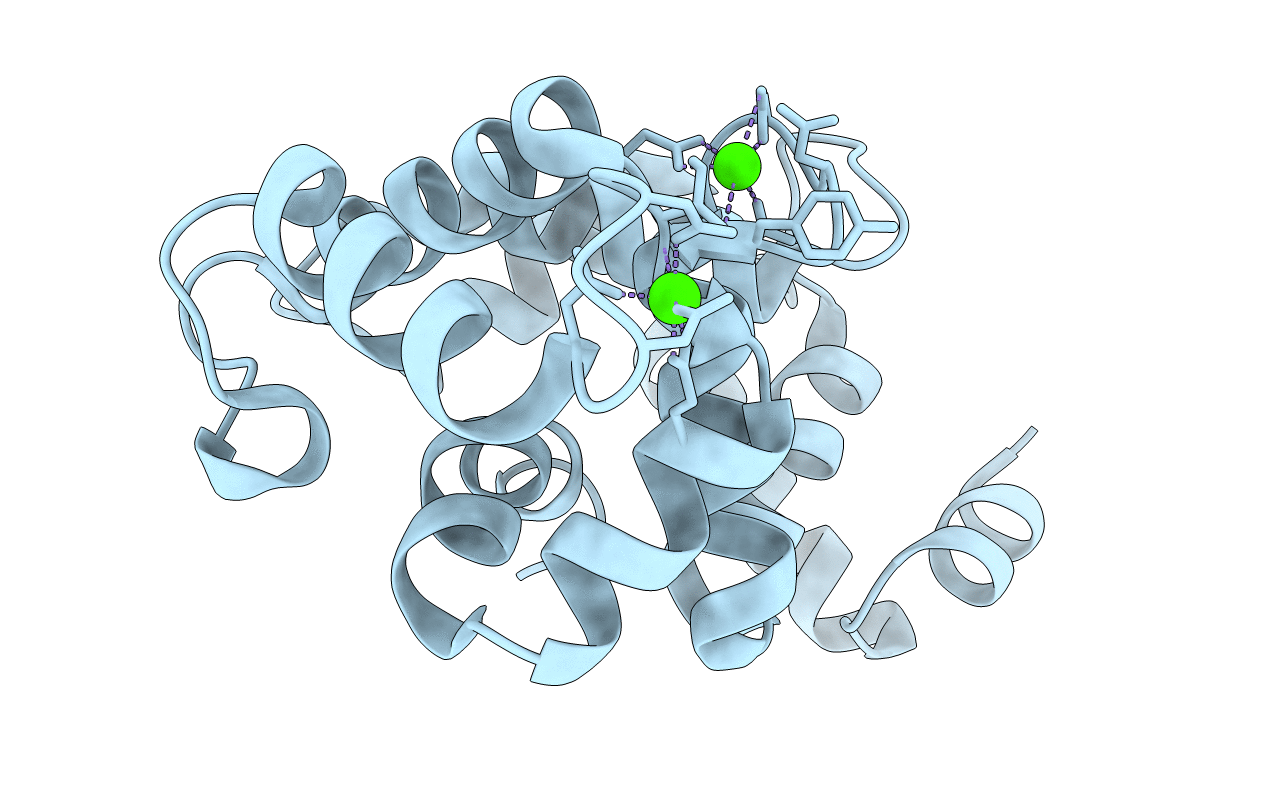
Deposition Date
2004-01-06
Release Date
2005-01-11
Last Version Date
2023-08-23
Entry Detail
PDB ID:
1S1E
Keywords:
Title:
Crystal Structure of Kv Channel-interacting protein 1 (KChIP-1)
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.30 Å
R-Value Free:
0.27
R-Value Work:
0.22
R-Value Observed:
0.25
Space Group:
P 41 21 2