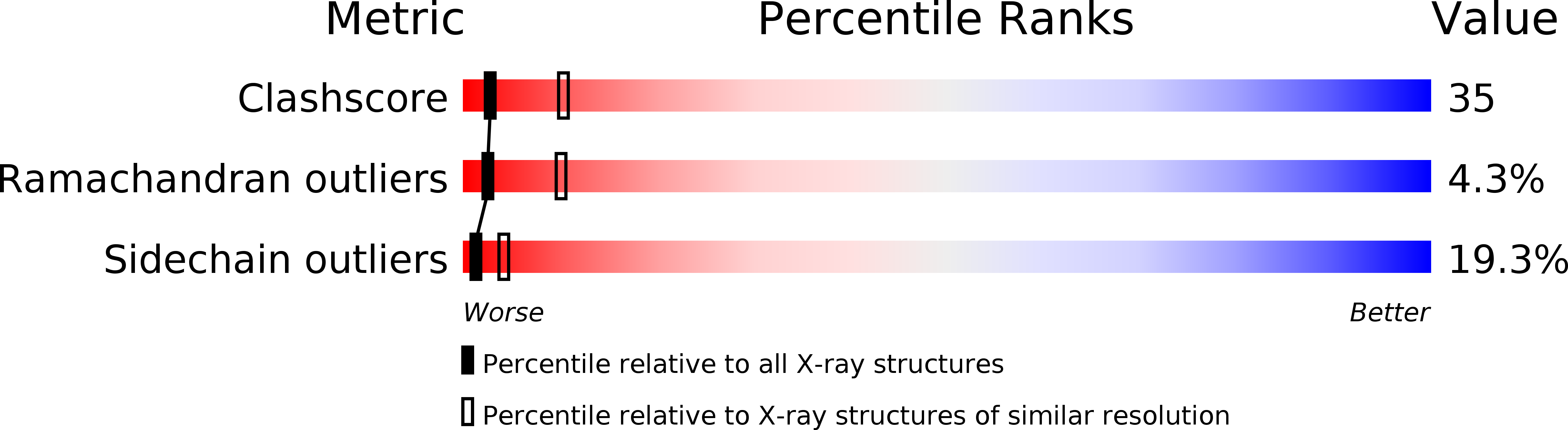Deposition Date
1991-10-10
Release Date
1991-10-15
Last Version Date
2024-02-14
Entry Detail
PDB ID:
1RUS
Keywords:
Title:
CRYSTAL STRUCTURE OF THE BINARY COMPLEX OF RIBULOSE-1,5-BISPHOSPHATE CARBOXYLASE AND ITS PRODUCT, 3-PHOSPHO-D-GLYCERATE
Biological Source:
Source Organism(s):
Rhodospirillum rubrum (Taxon ID: 1085)
Method Details:
Experimental Method:
Resolution:
2.90 Å
R-Value Observed:
0.20
Space Group:
P 1 21 1