Deposition Date
2003-12-04
Release Date
2004-06-08
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1RQ1
Keywords:
Title:
Structure of Ero1p, Source of Disulfide Bonds for Oxidative Protein Folding in the Cell
Biological Source:
Source Organism:
Saccharomyces cerevisiae (Taxon ID: 4932)
Host Organism:
Method Details:
Experimental Method:
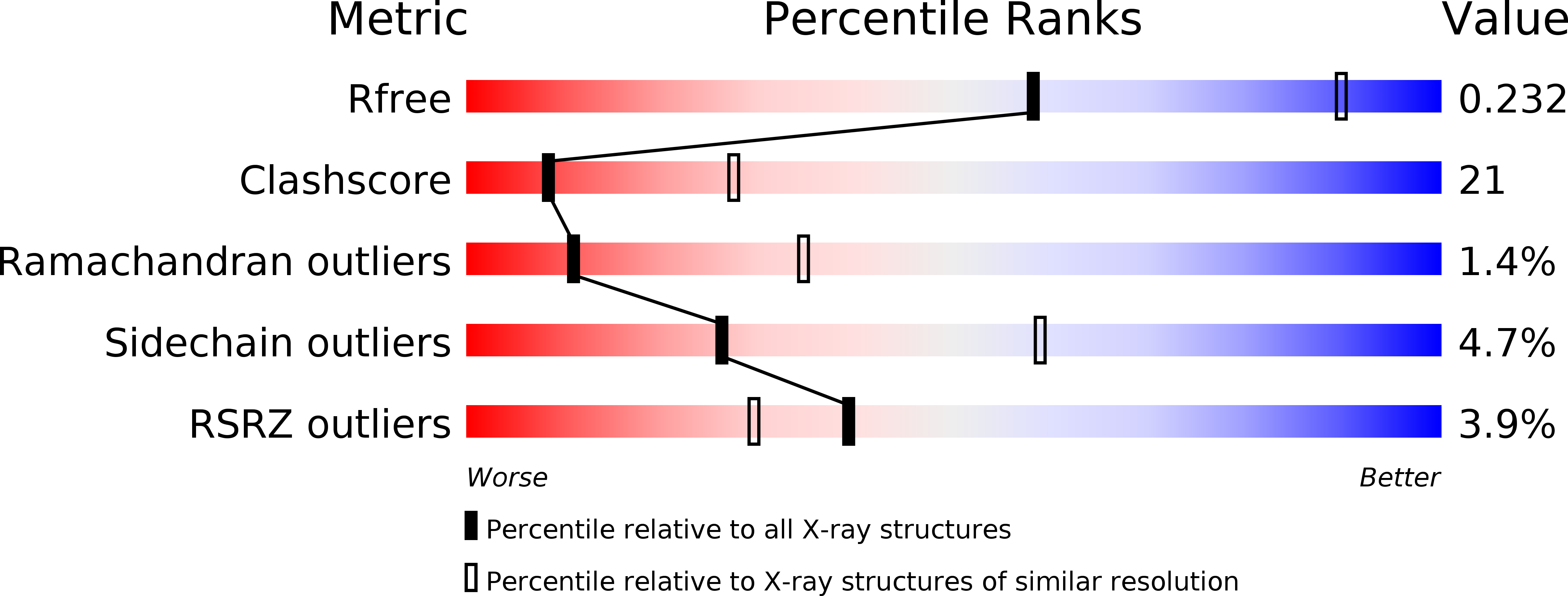
Resolution:
2.80 Å
R-Value Free:
0.23
R-Value Work:
0.20
Space Group:
P 62