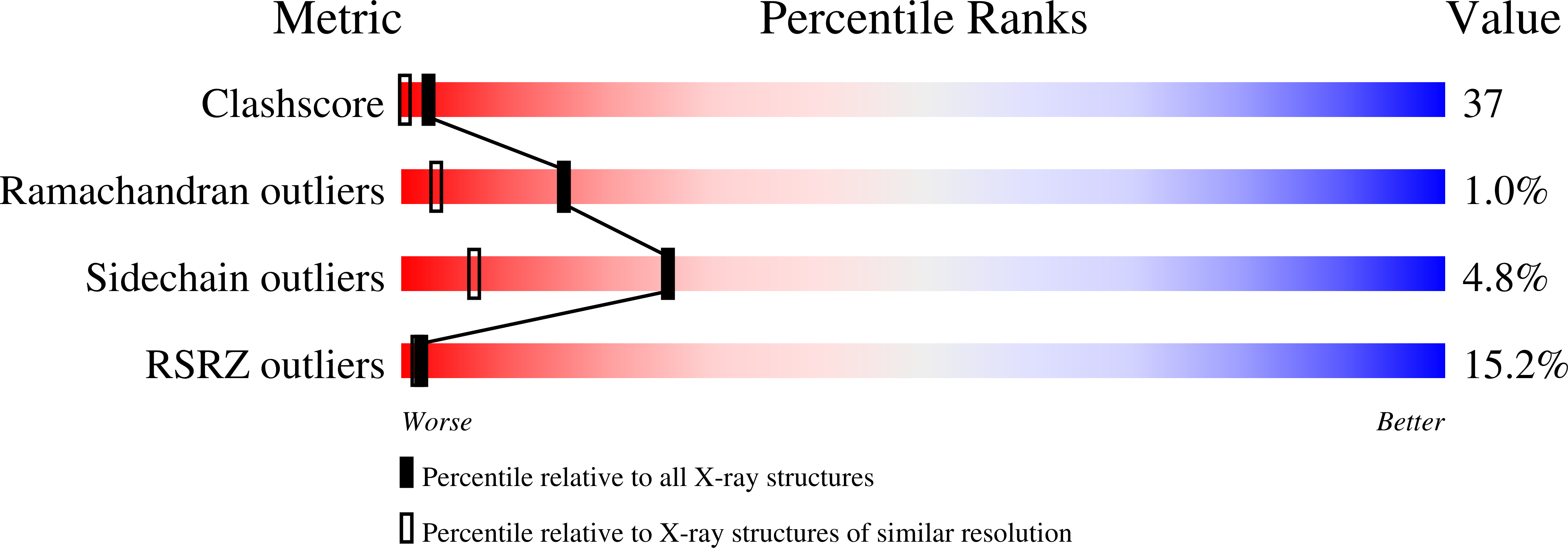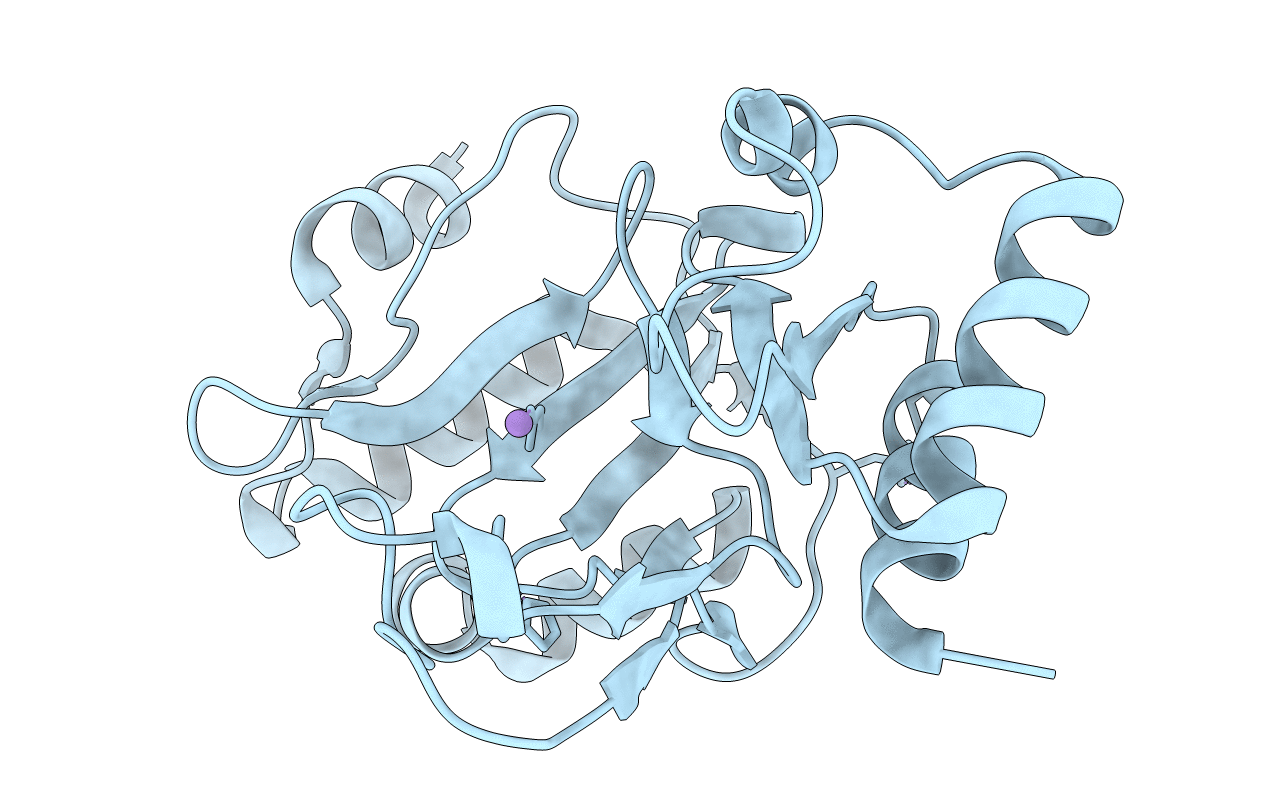
Deposition Date
2003-12-01
Release Date
2004-01-27
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1RO2
Keywords:
Title:
Bifunctional DNA primase/polymerase domain of ORF904 from the archaeal plasmid pRN1- Triple mutant F50M/L107M/L110M manganese soak
Biological Source:
Source Organism(s):
Sulfolobus islandicus (Taxon ID: 43080)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.60 Å
R-Value Free:
0.26
R-Value Work:
0.25
Space Group:
P 21 21 2