Deposition Date
2003-11-20
Release Date
2004-12-07
Last Version Date
2024-02-14
Entry Detail
PDB ID:
1RJU
Keywords:
Title:
Crystal structure of a truncated form of yeast copper thionein
Method Details:
Experimental Method:
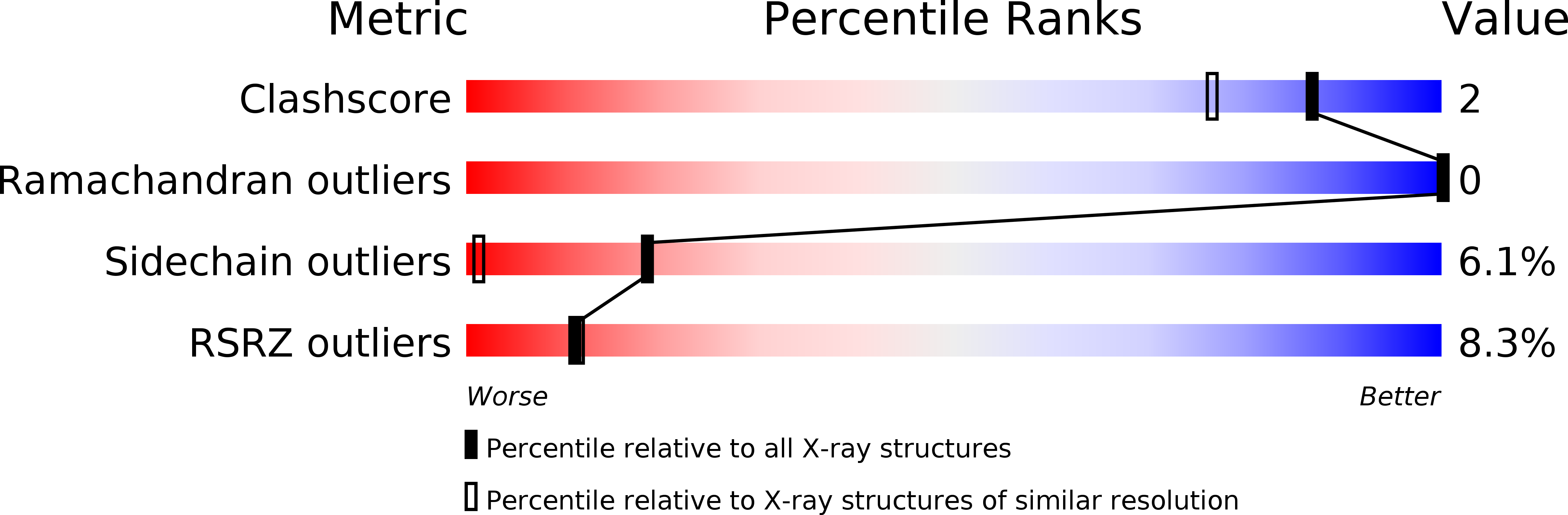
Resolution:
1.44 Å
R-Value Free:
0.17
R-Value Work:
0.14
R-Value Observed:
0.14
Space Group:
P 43 3 2