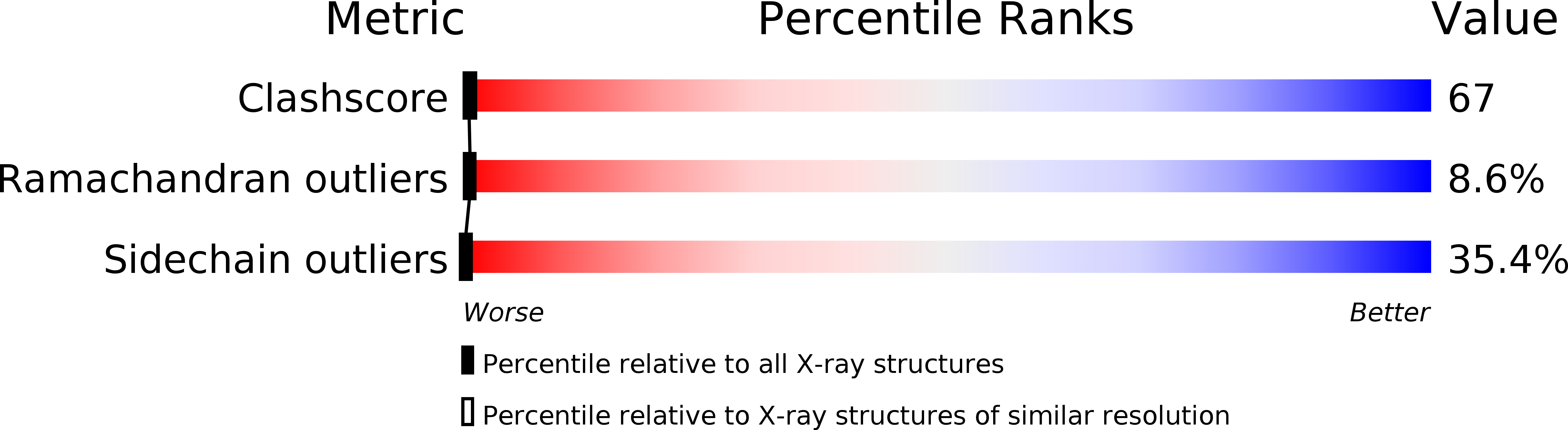
Deposition Date
1991-11-18
Release Date
1994-01-31
Last Version Date
2024-02-14
Entry Detail
PDB ID:
1RBA
Keywords:
Title:
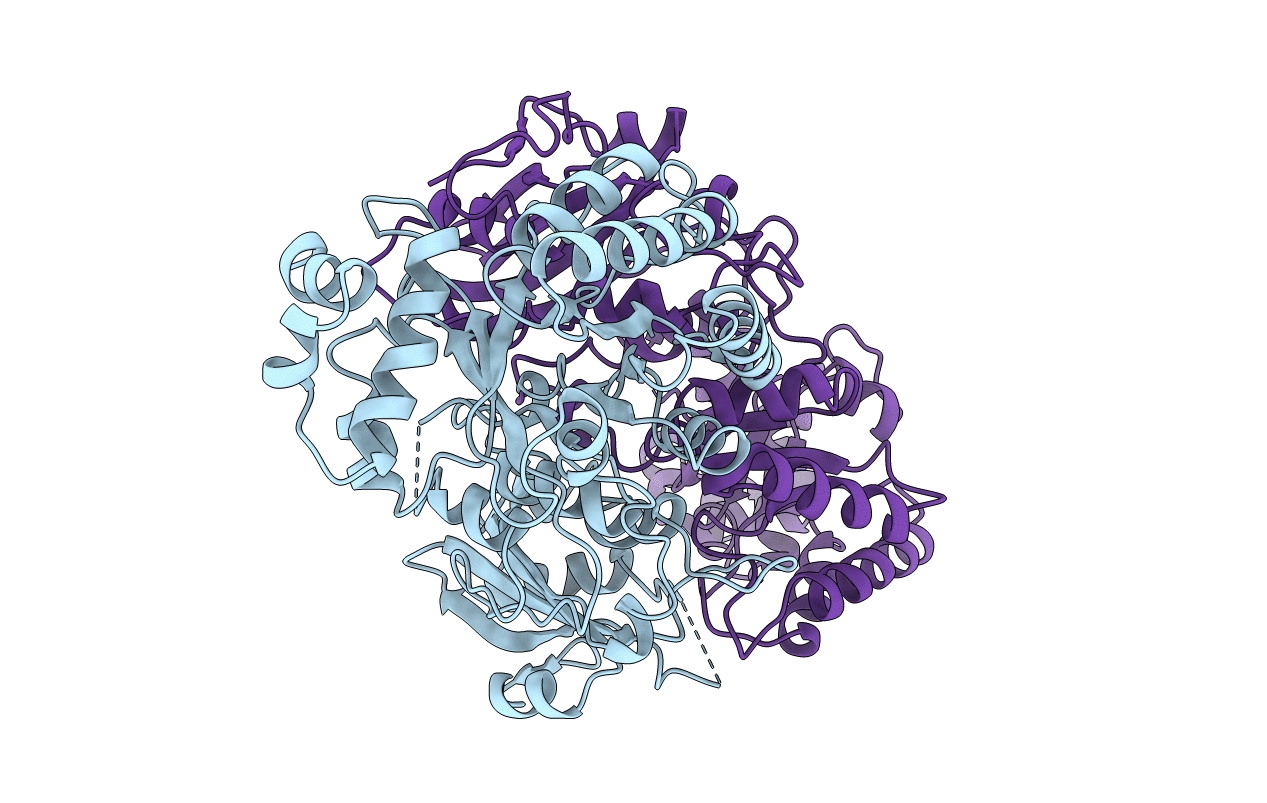
SUBSTITUTION OF ASP193 TO ASN AT THE ACTIVE SITE OF RIBULOSE-1,5-BISPHOSPHATE CARBOXYLASE RESULTS IN CONFORMATIONAL CHANGES
Biological Source:
Source Organism(s):
Rhodospirillum rubrum (Taxon ID: 1085)
Method Details:
Experimental Method:
Resolution:
2.60 Å
R-Value Observed:
0.20
Space Group:
P 1 21 1