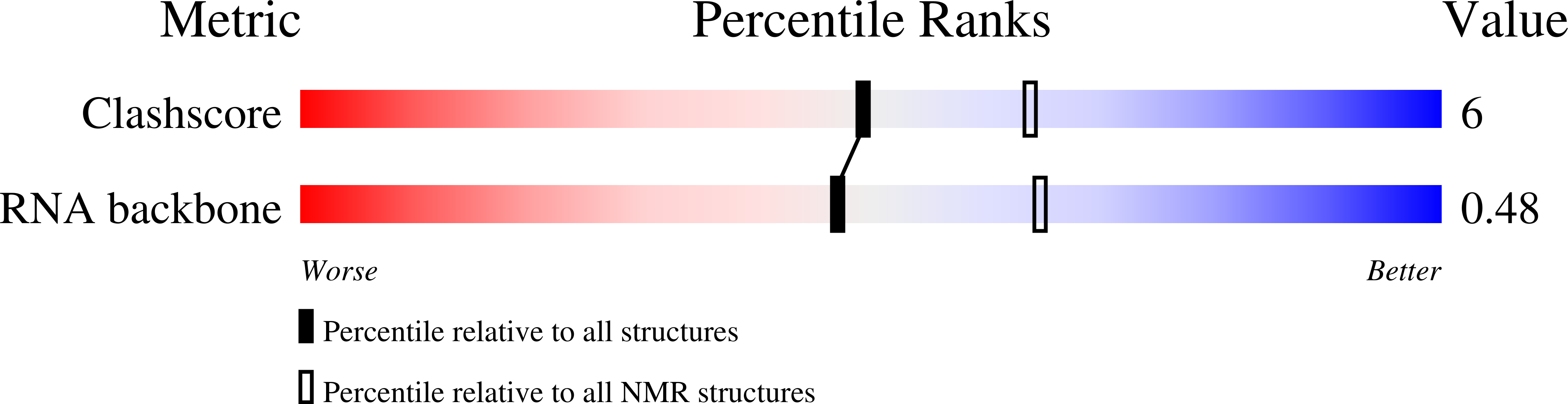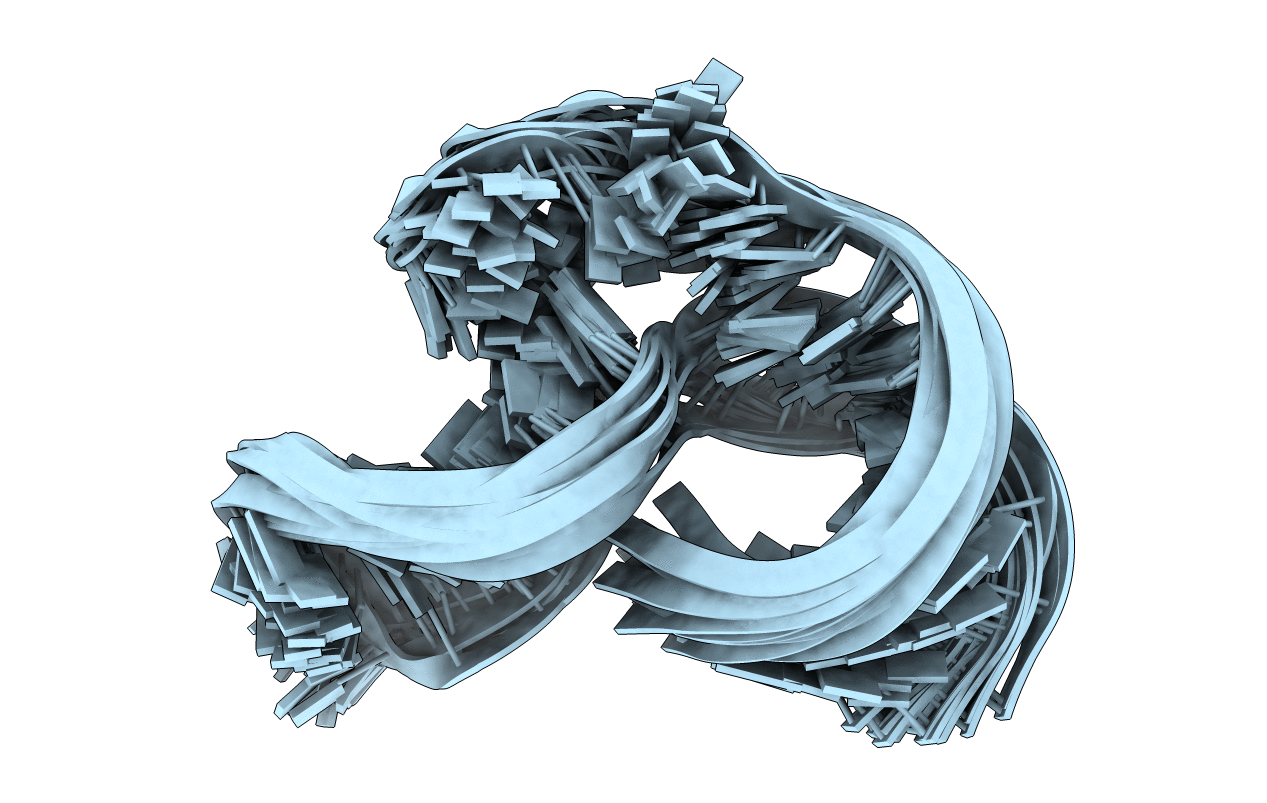
Deposition Date
2003-10-22
Release Date
2004-05-25
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1R7Z
Keywords:
Title:
NMR STRUCTURE OF THE R(GGAGGACAUUCCUCACGGGUGACCGUGGUCCUCC), DOMAIN IV STEM-LOOP B OF ENTEROVIRAL IRES WITH AUUCCU BULGE
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
20
Selection Criteria:
LOWEST TOTAL ENERGY (A WEIGHTED SUM OF CONFORMATIONAL ENERGY AND RESTRAINT ENERGY)