Deposition Date
2003-09-05
Release Date
2003-09-16
Last Version Date
2024-11-06
Entry Detail
PDB ID:
1QXD
Keywords:
Title:
Structural Basis for the Potent Antisickling Effect of a Novel Class of 5-Membered Heterocyclic Aldehydic Compounds
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Method Details:
Experimental Method:
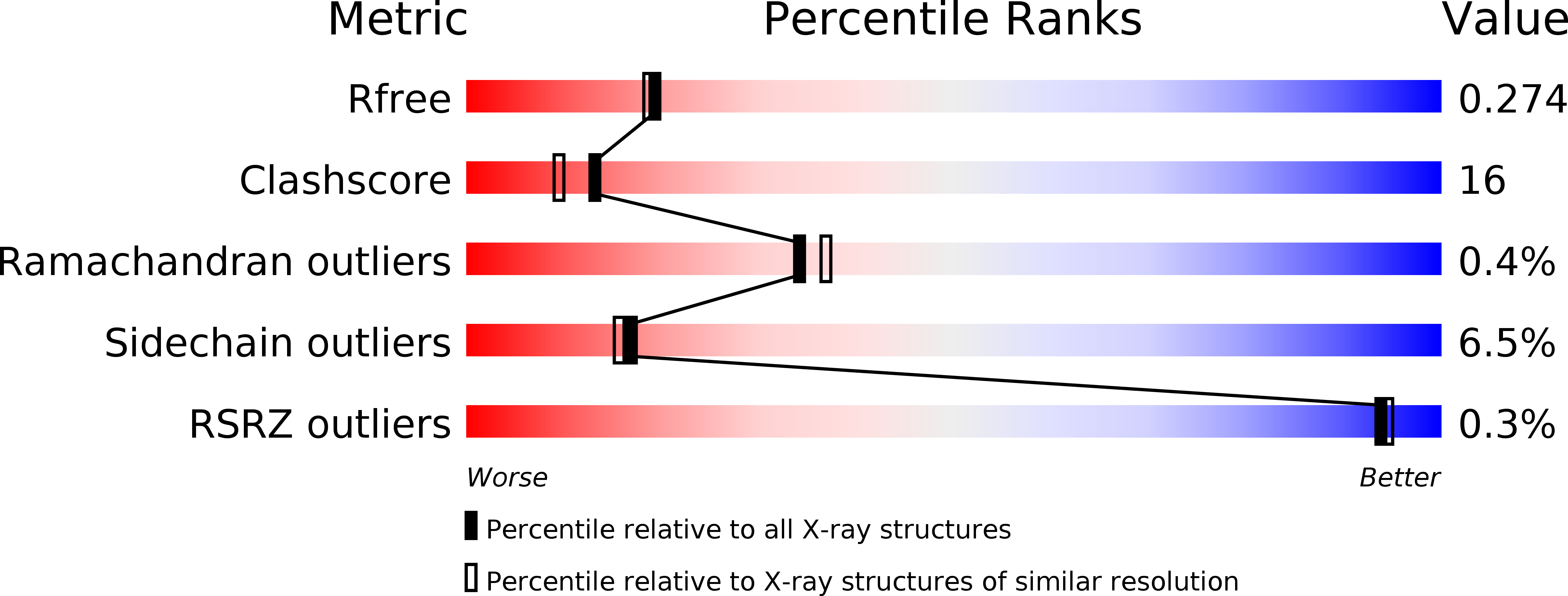
Resolution:
2.25 Å
R-Value Free:
0.27
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 32 2 1