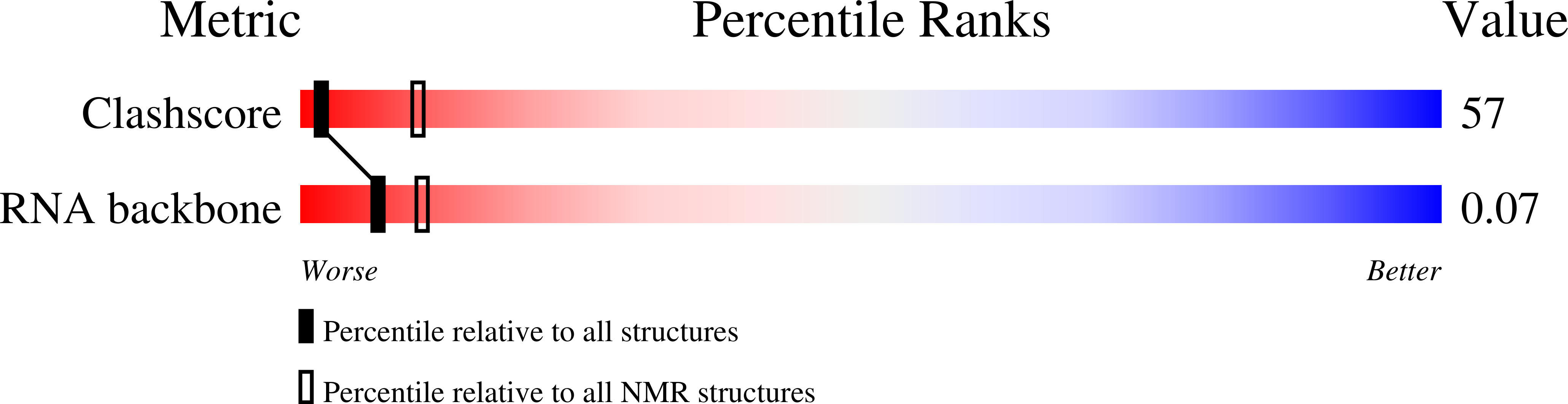Deposition Date
2003-09-01
Release Date
2003-11-25
Last Version Date
2024-05-01
Entry Detail
PDB ID:
1QWA
Keywords:
Title:
NMR structure of 5'-r(GGAUGCCUCCCGAGUGCAUCC): an RNA hairpin derived from the mouse 5'ETS that binds nucleolin RBD12.
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Method Details:
Experimental Method:
Conformers Calculated:
17
Conformers Submitted:
17
Selection Criteria:
all calculated structures submitted,back calculated data agree with experimental NOESY spectrum,structures with acceptable covalent geometry,structures with the least restraint violations,structures with the lowest energy