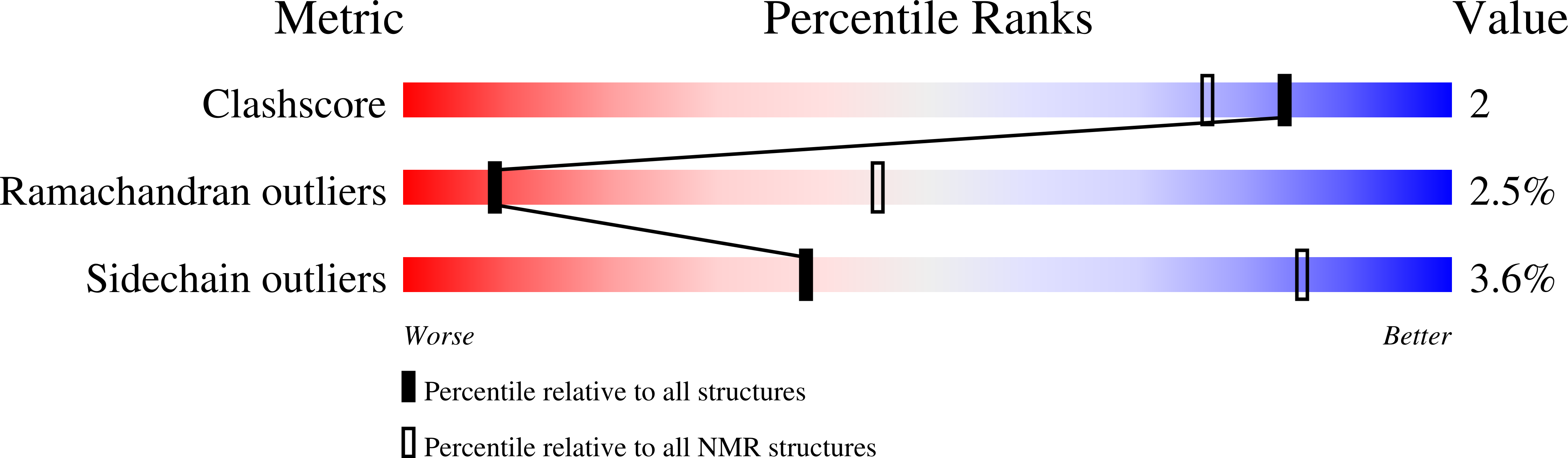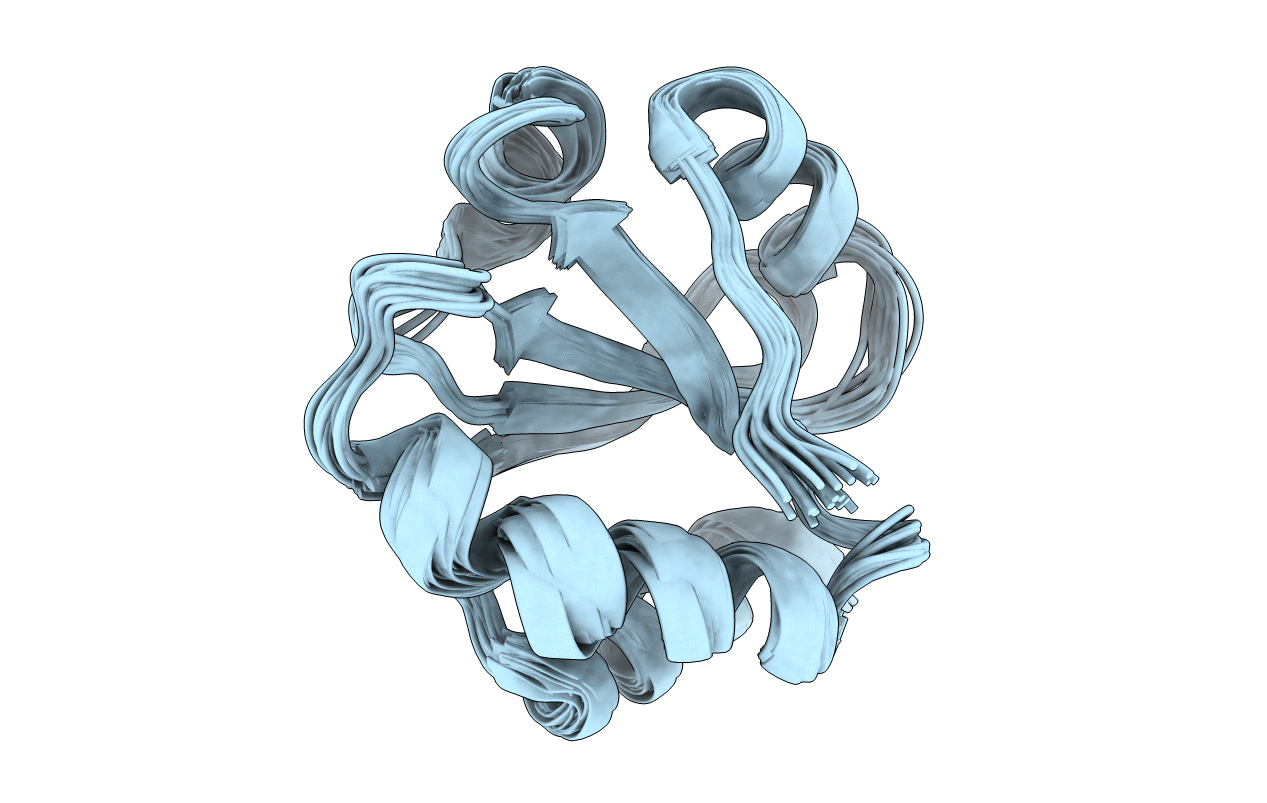
Deposition Date
1999-07-02
Release Date
2000-01-26
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1QUW
Keywords:
Title:
SOLUTION STRUCTURE OF THE THIOREDOXIN FROM BACILLUS ACIDOCALDARIUS
Biological Source:
Source Organism:
Alicyclobacillus acidocaldarius (Taxon ID: 405212)
Host Organism:
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
20
Selection Criteria:
STRUCTURES WITH ACCEPTABLE COVALENT GEOMETRY,STRUCTURES WITH THE LEAST
RESTRAINT VIOLATIONS