Deposition Date
1999-07-06
Release Date
1999-12-17
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1QU5
Keywords:
Title:
NMR STRUCTURE OF A NEW PHOSPHOTYROSINE BINDING DOMAIN CONTAINING THE FHA2 DOMAIN OF RAD 53
Biological Source:
Source Organism:
Saccharomyces cerevisiae (Taxon ID: 4932)
Host Organism:
Method Details:
Experimental Method:
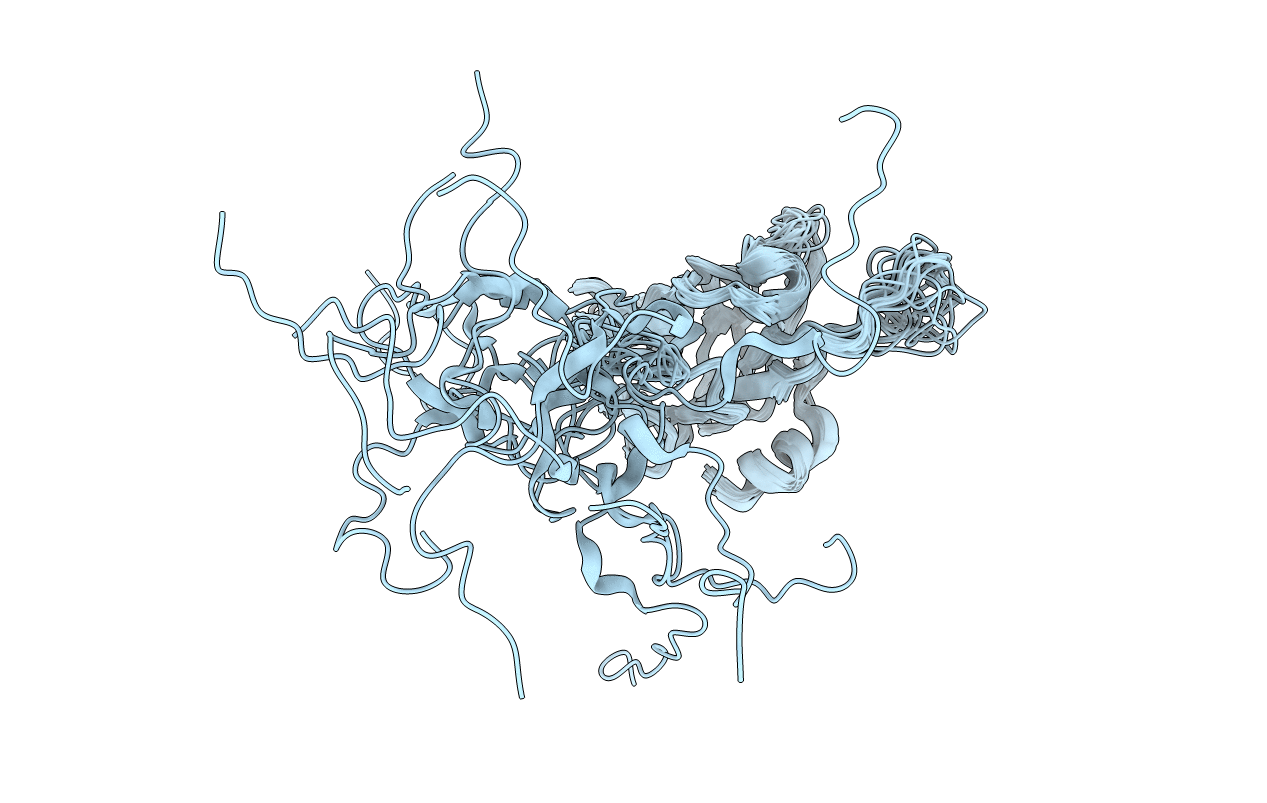
Conformers Calculated:
32
Conformers Submitted:
16
Selection Criteria:
structures with the lowest energy