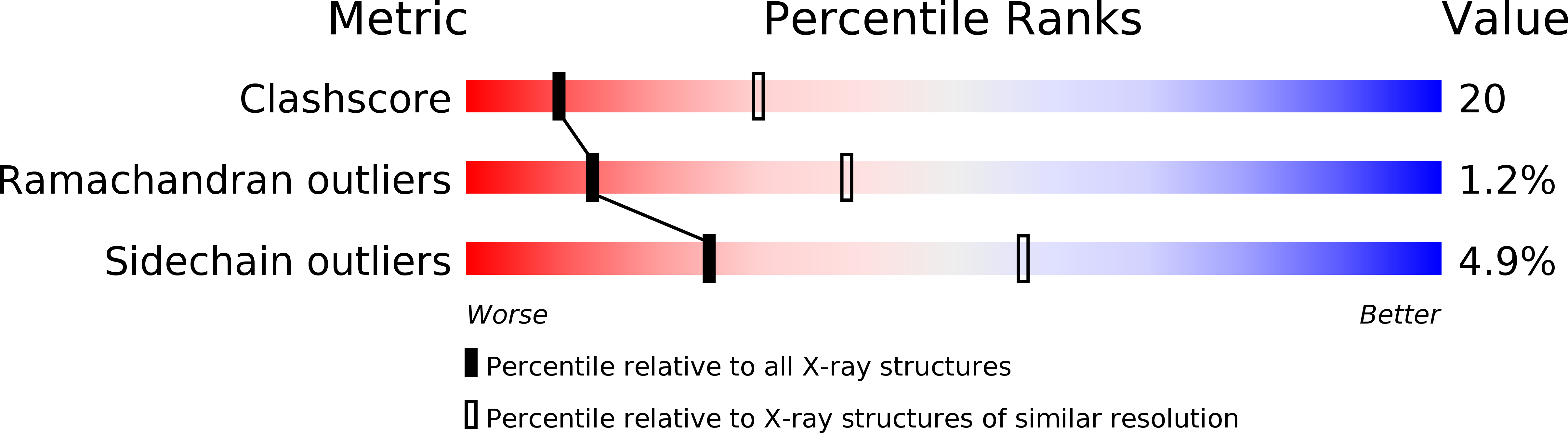Deposition Date
1999-06-14
Release Date
1999-10-14
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1QRN
Keywords:
Title:
CRYSTAL STRUCTURE OF HUMAN A6 TCR COMPLEXED WITH HLA-A2 BOUND TO ALTERED HTLV-1 TAX PEPTIDE P6A
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.80 Å
R-Value Free:
0.27
R-Value Work:
0.21
Space Group:
C 1 2 1