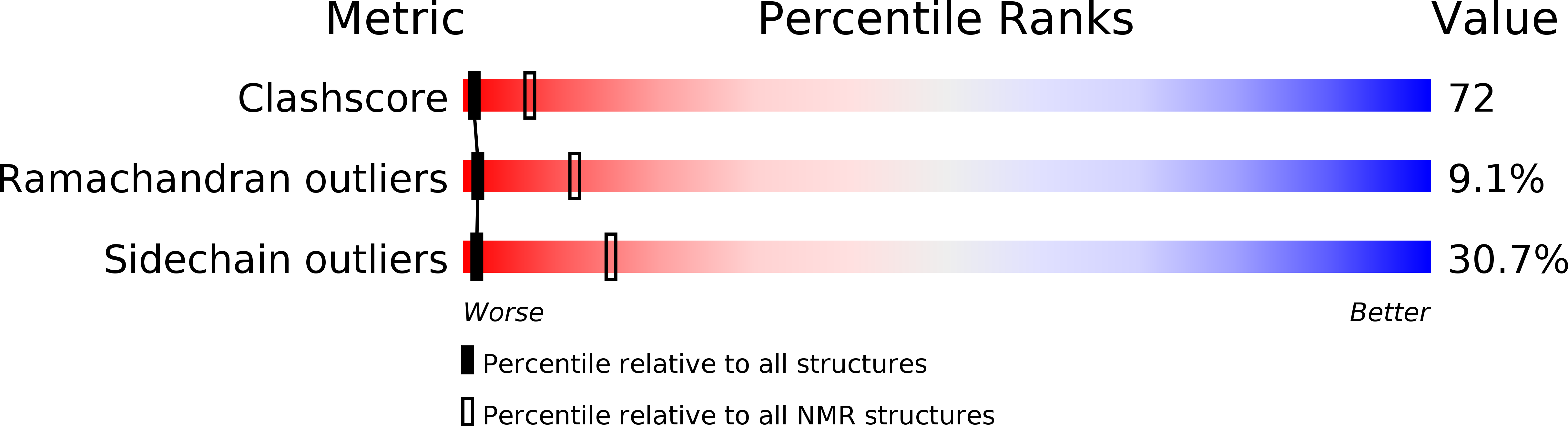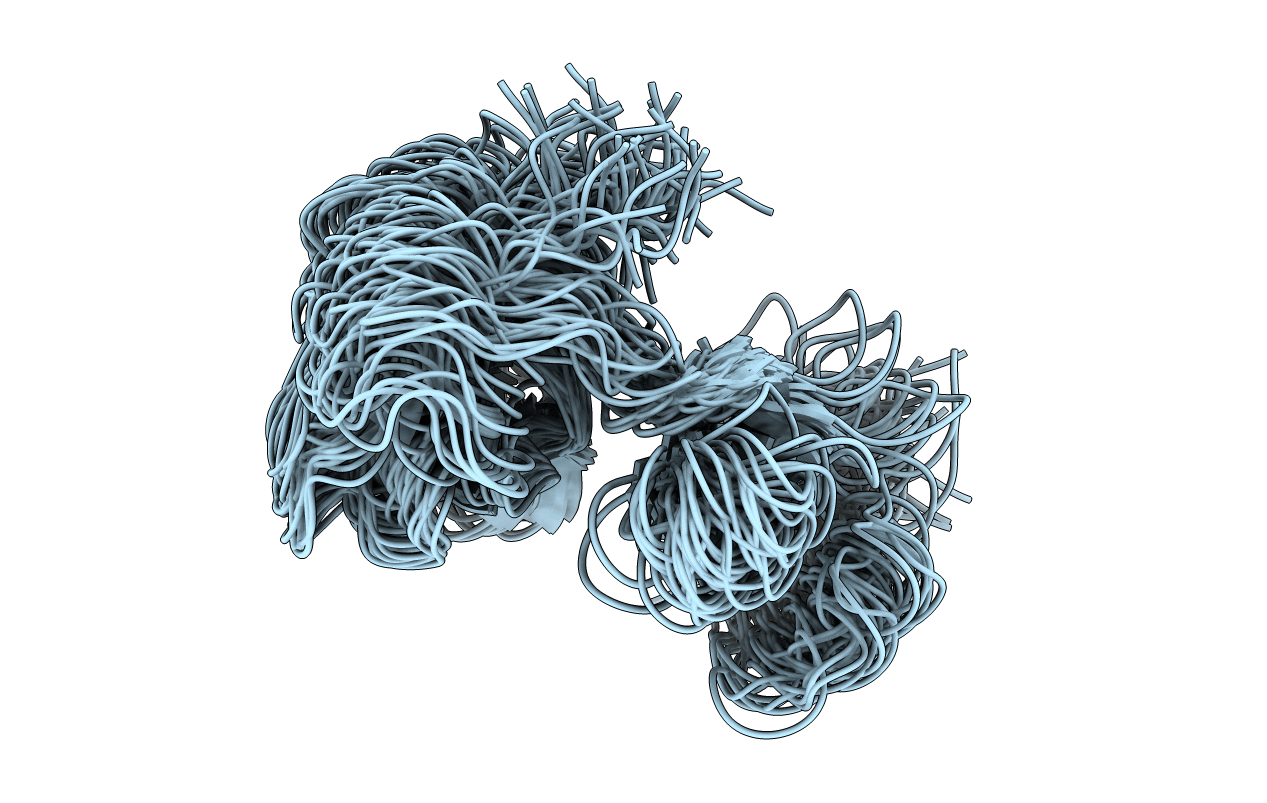
Deposition Date
1999-11-04
Release Date
2000-01-11
Last Version Date
2024-11-13
Entry Detail
PDB ID:
1QO6
Keywords:
Title:
Solution structure of a pair of modules from the gelatin-binding domain of fibronectin
Biological Source:
Source Organism:
HOMO SAPIENS (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
55
Selection Criteria:
LOW ENERGY AND AGREEMENT WITH EXPERIMENTAL RESTRAINTS