Deposition Date
1999-09-28
Release Date
2000-03-31
Last Version Date
2023-12-13
Entry Detail
PDB ID:
1QMG
Keywords:
Title:
Acetohydroxyacid isomeroreductase complexed with its reaction product dihydroxy-methylvalerate, manganese and ADP-ribose.
Biological Source:
Source Organism:
SPINACIA OLERACEA (Taxon ID: 3562)
Host Organism:
Method Details:
Experimental Method:
Resolution:
1.60 Å
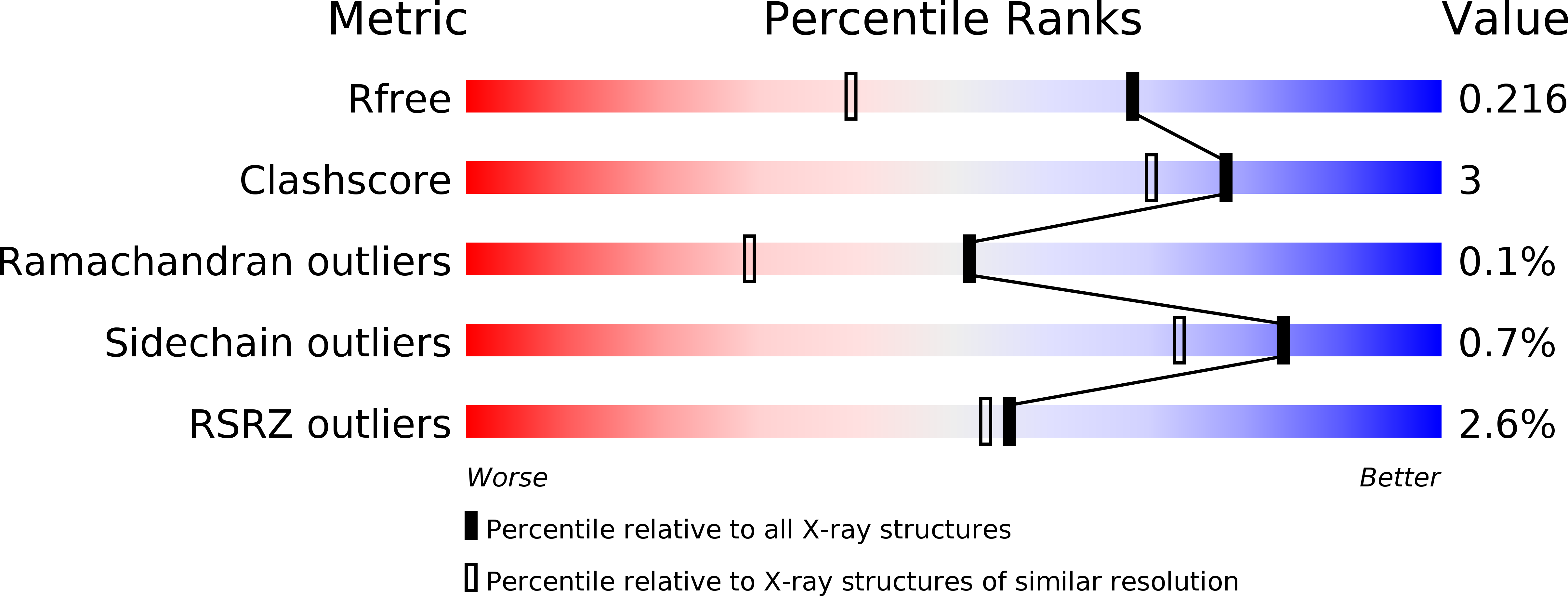
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 1 21 1