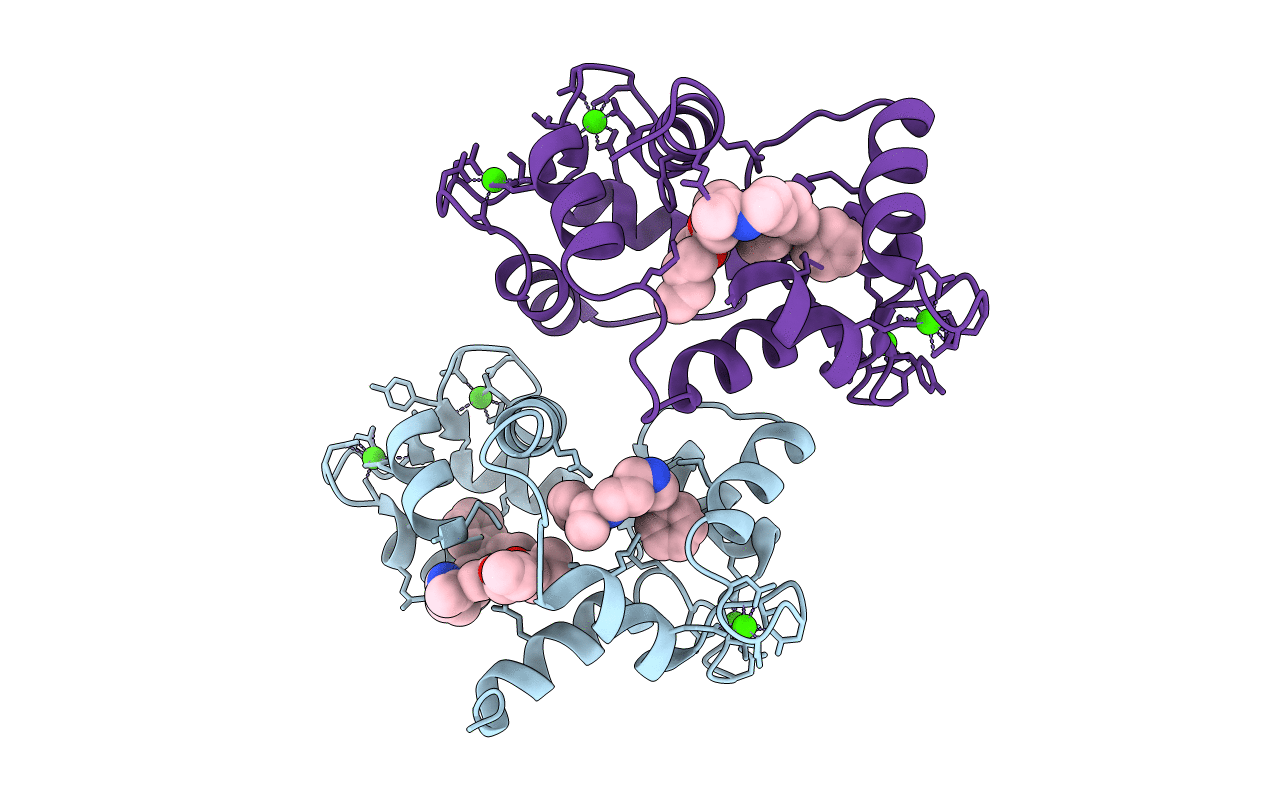
Deposition Date
1999-06-17
Release Date
2000-03-28
Last Version Date
2023-12-13
Entry Detail
PDB ID:
1QIW
Keywords:
Title:
Calmodulin complexed with N-(3,3,-diphenylpropyl)-N'-[1-R-(3,4-bis-butoxyphenyl)-ethyl]-propylenediamine (DPD)
Biological Source:
Source Organism:
BOS TAURUS (Taxon ID: 9913)
Method Details:
Experimental Method:
Resolution:
2.30 Å
R-Value Free:
0.31
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 1


