Deposition Date
1999-06-16
Release Date
1999-10-29
Last Version Date
2024-02-07
Entry Detail
PDB ID:
1QIU
Keywords:
Title:
A triple beta-spiral in the adenovirus fibre shaft reveals a new structural motif for biological fibres
Biological Source:
Source Organism:
HUMAN ADENOVIRUS 2 (Taxon ID: 10515)
Host Organism:
Method Details:
Experimental Method:
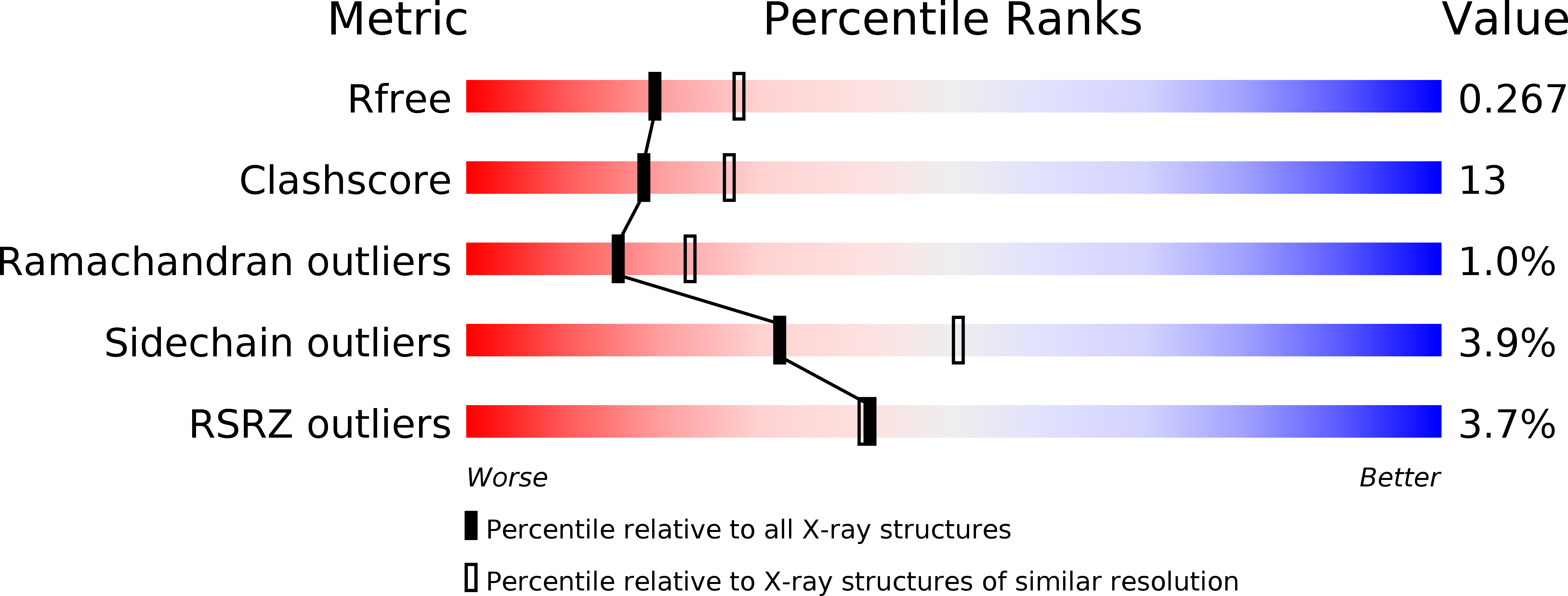
Resolution:
2.40 Å
R-Value Free:
0.26
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
C 1 2 1