Deposition Date
1999-05-11
Release Date
2000-05-17
Last Version Date
2024-11-13
Entry Detail
PDB ID:
1QH7
Keywords:
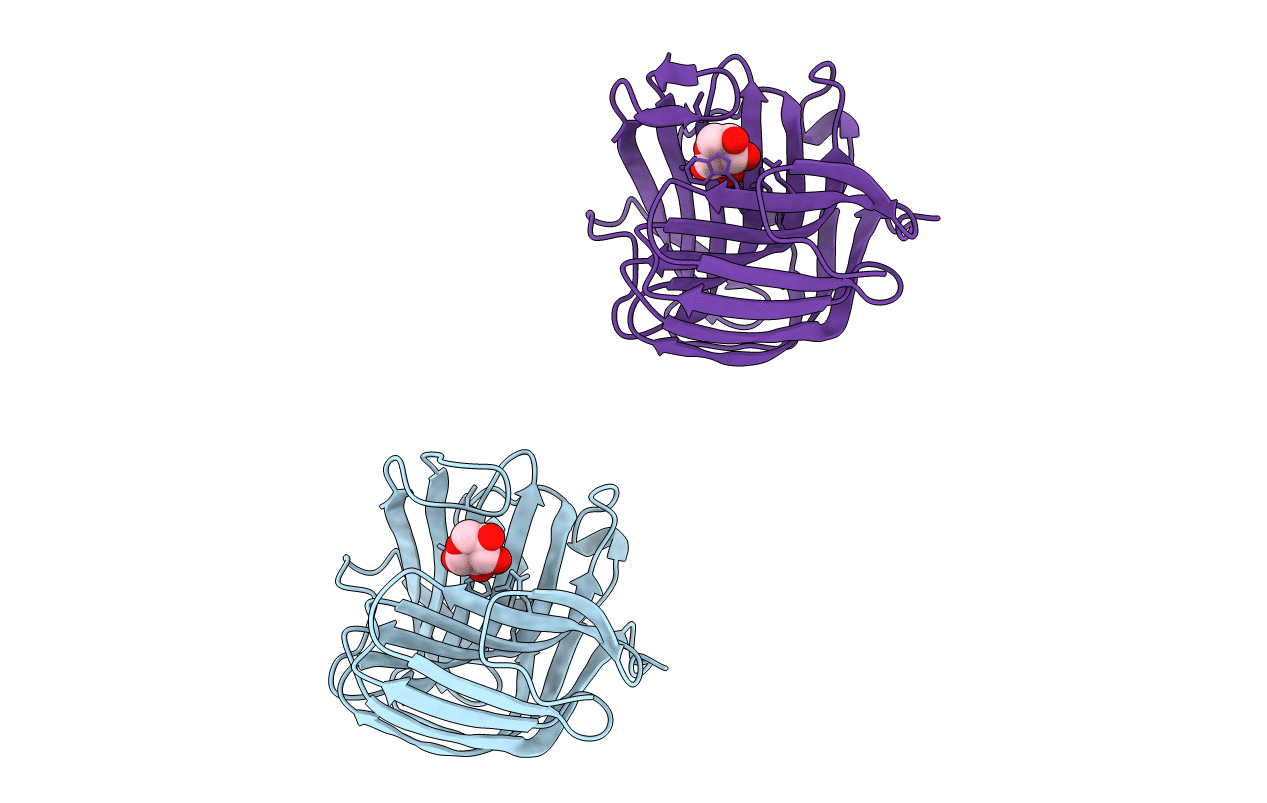
Title:
CATALYSIS AND SPECIFICITY IN ENZYMATIC GLYCOSIDE HYDROLASES: A 2,5B CONFORMATION FOR THE GLYCOSYL-ENZYME INTERMIDIATE REVEALED BY THE STRUCTURE OF THE BACILLUS AGARADHAERENS FAMILY 11 XYLANASE
Biological Source:
Source Organism(s):
Bacillus agaradhaerens (Taxon ID: 76935)
Expression System(s):
Method Details:
Experimental Method:
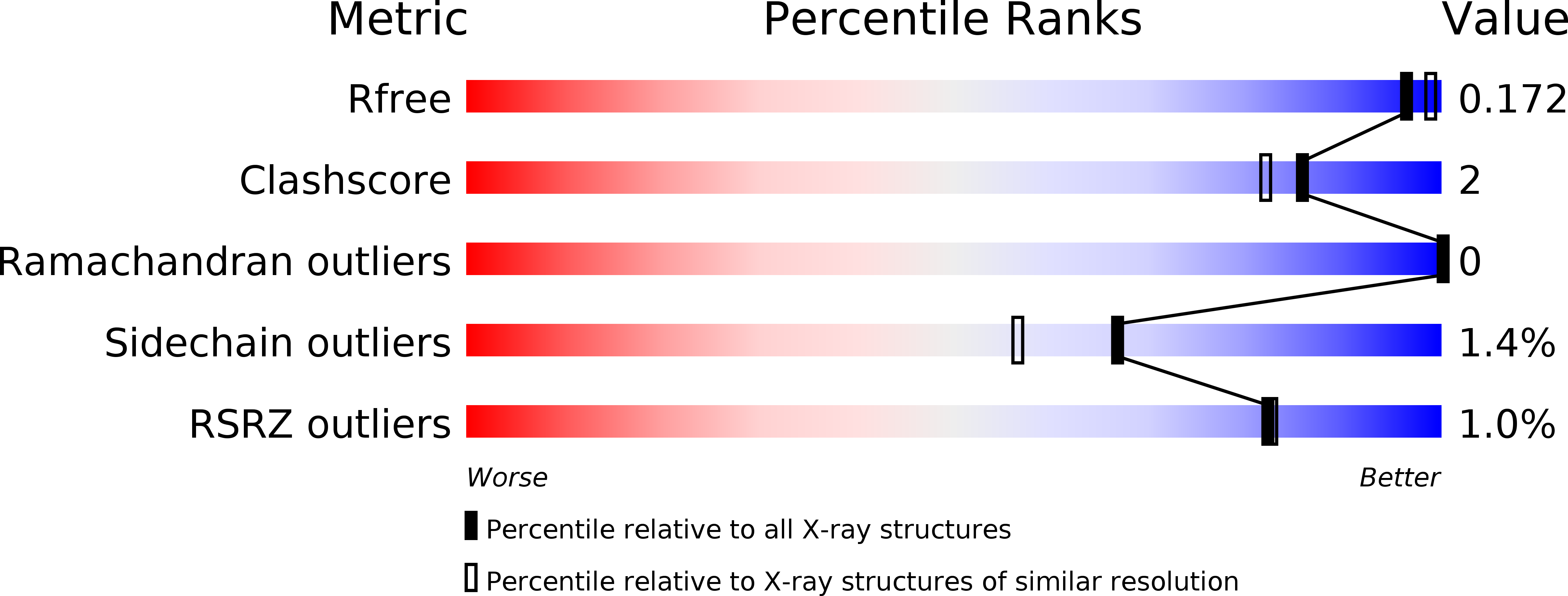
Resolution:
1.78 Å
R-Value Free:
0.17
R-Value Work:
0.11
Space Group:
P 21 21 21