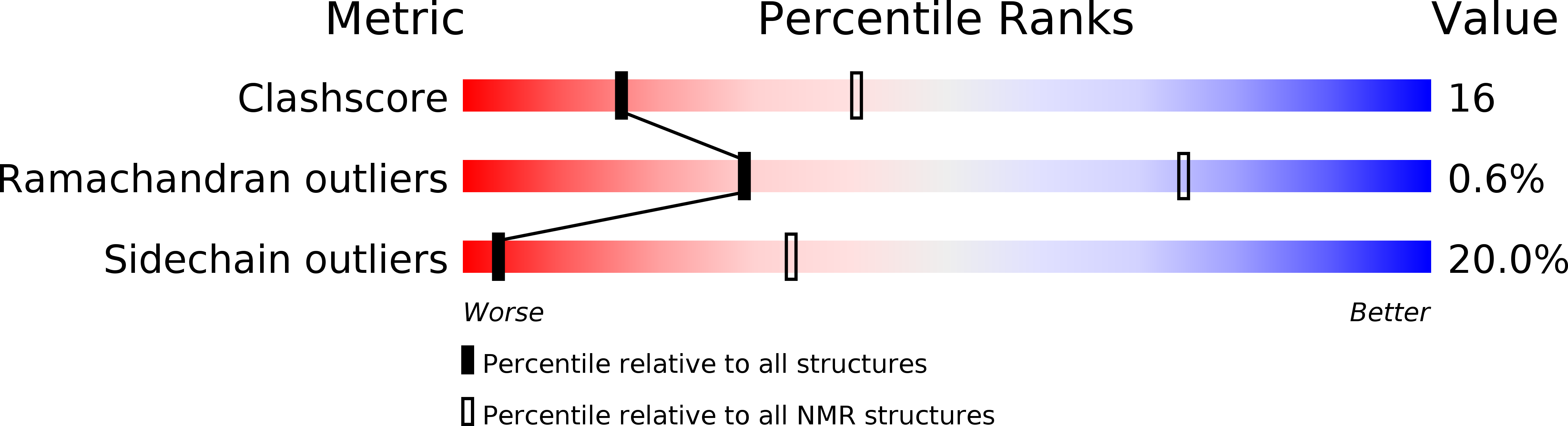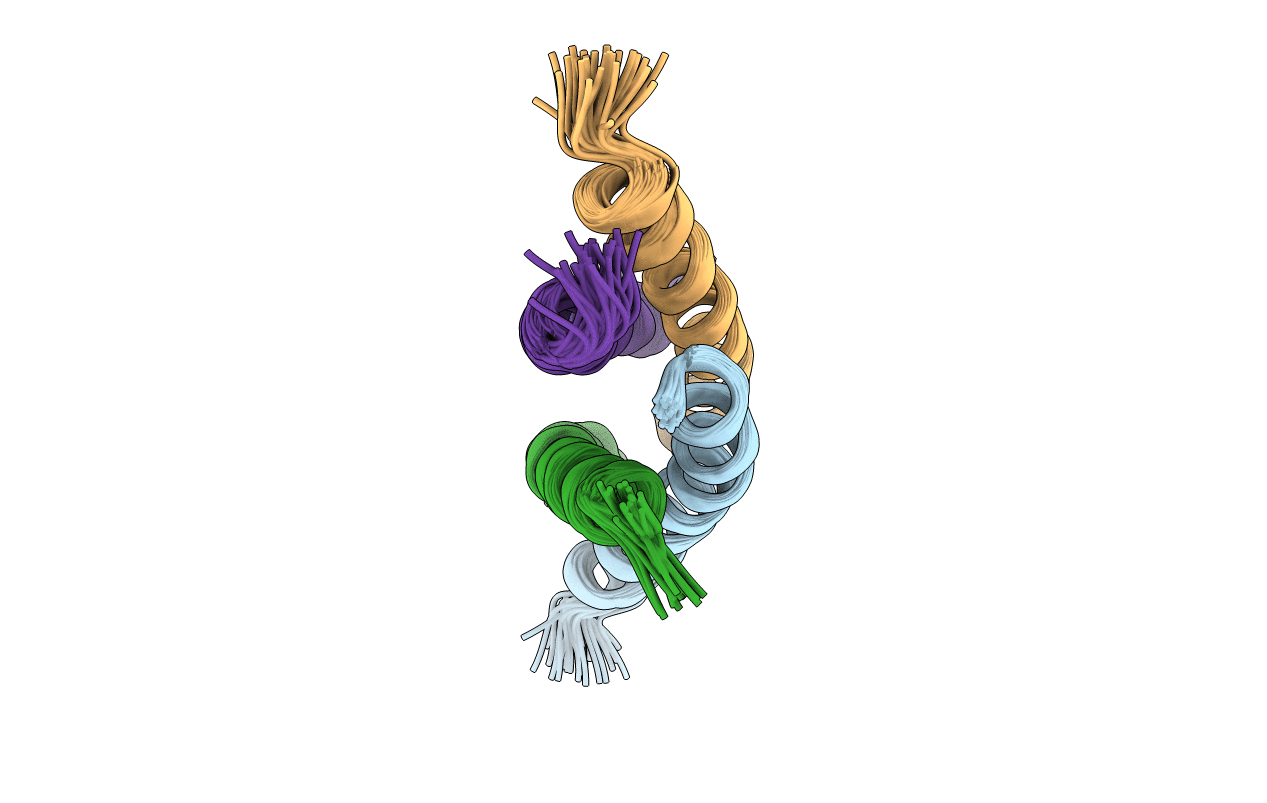
Deposition Date
1999-04-03
Release Date
1999-08-18
Last Version Date
2023-12-27
Entry Detail
PDB ID:
1QEY
Keywords:
Title:
NMR Structure Determination of the Tetramerization Domain of the MNT Repressor: An Asymmetric A-Helical Assembly in Slow Exchange
Biological Source:
Source Organism(s):
Enterobacteria phage P22 (Taxon ID: 10754)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
30
Conformers Submitted:
27
Selection Criteria:
LOWEST ENERGY, LEAST RESTRAINT VIOLATION