Deposition Date
2003-07-25
Release Date
2004-01-13
Last Version Date
2024-04-03
Entry Detail
PDB ID:
1Q2O
Keywords:
Title:
Bovine endothelial nitric oxide synthase N368D mutant heme domain dimer with L-N(omega)-nitroarginine-2,4-L-diaminobutyramide bound
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Expression System(s):
Method Details:
Experimental Method:
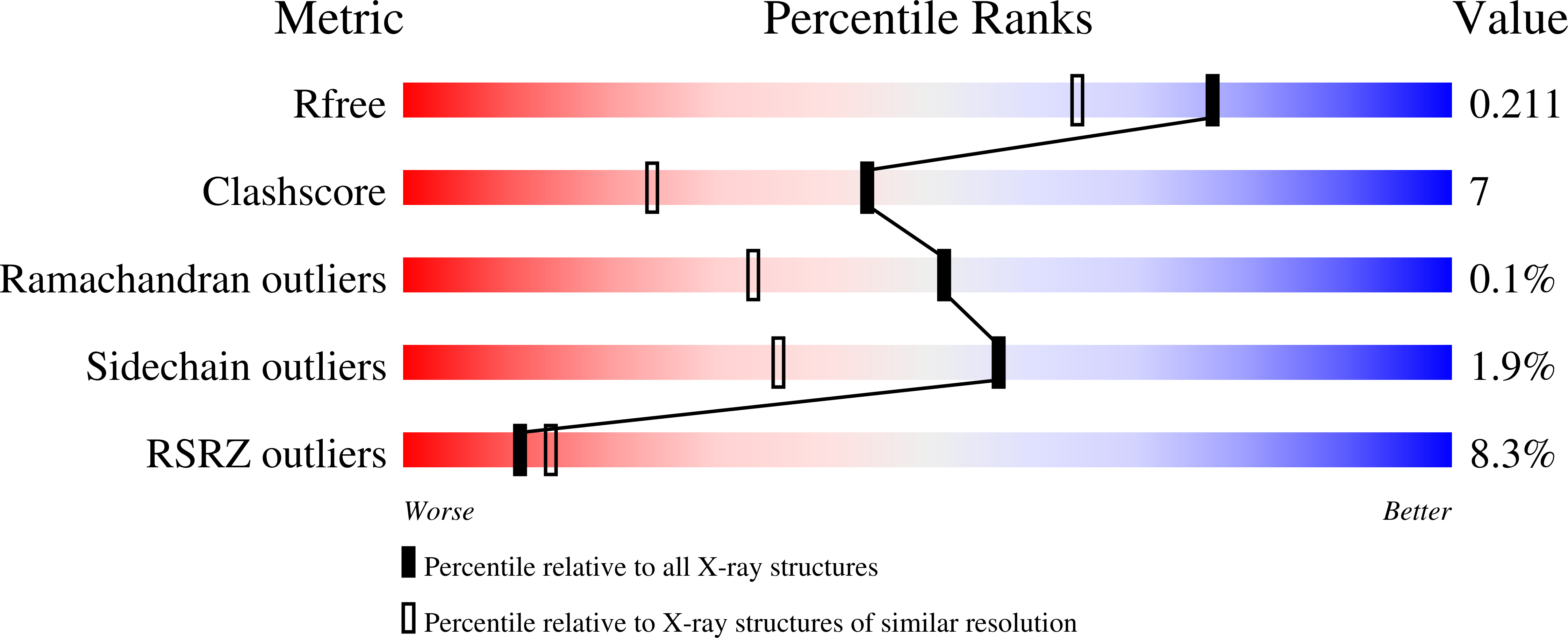
Resolution:
1.74 Å
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 21 21 21