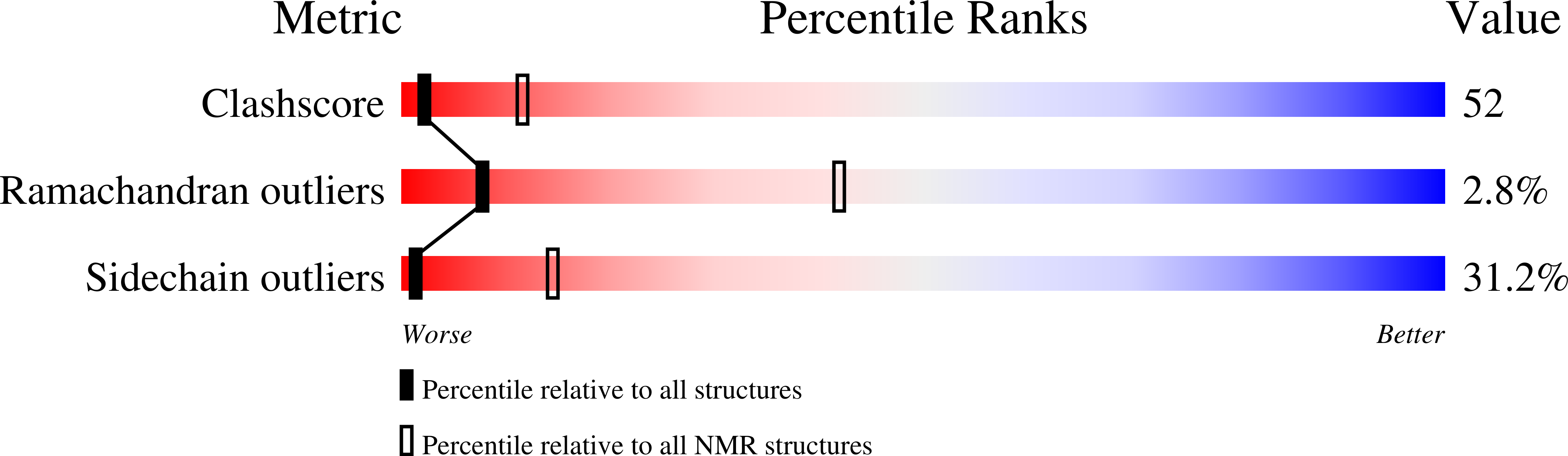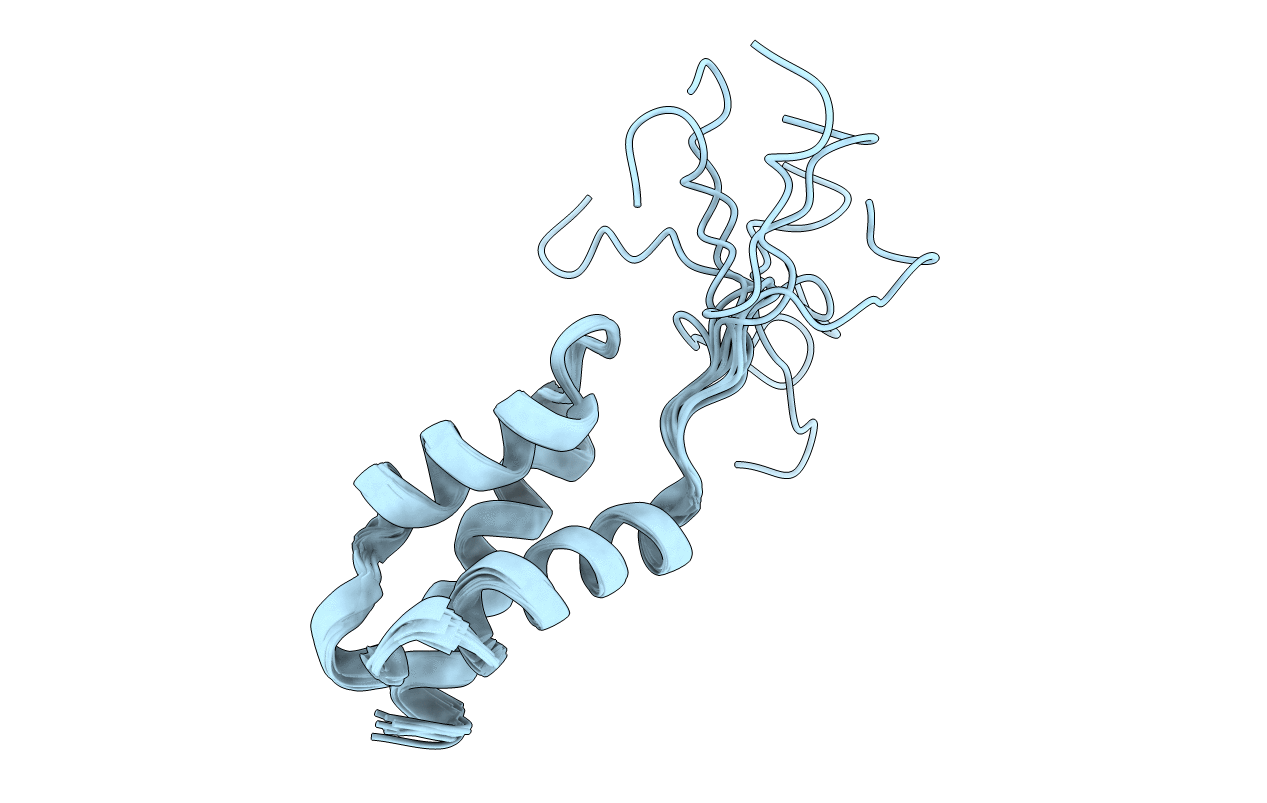
Deposition Date
2003-07-22
Release Date
2004-08-10
Last Version Date
2024-05-29
Entry Detail
PDB ID:
1Q1V
Keywords:
Title:
Structure of the Oncoprotein DEK: a putative DNA-binding Domain Related to the Winged Helix Motif
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
Conformers Calculated:
80
Conformers Submitted:
10
Selection Criteria:
STRUCTURES WITH THE LOWEST TOTAL ENERGY