Deposition Date
2003-07-14
Release Date
2003-09-16
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1PZT
Keywords:
Title:
CRYSTAL STRUCTURE OF W314A-BETA-1,4-GALACTOSYLTRANSFERASE (B4GAL-T1) CATALYTIC DOMAIN WITHOUT SUBSTRATE
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Expression System(s):
Method Details:
Experimental Method:
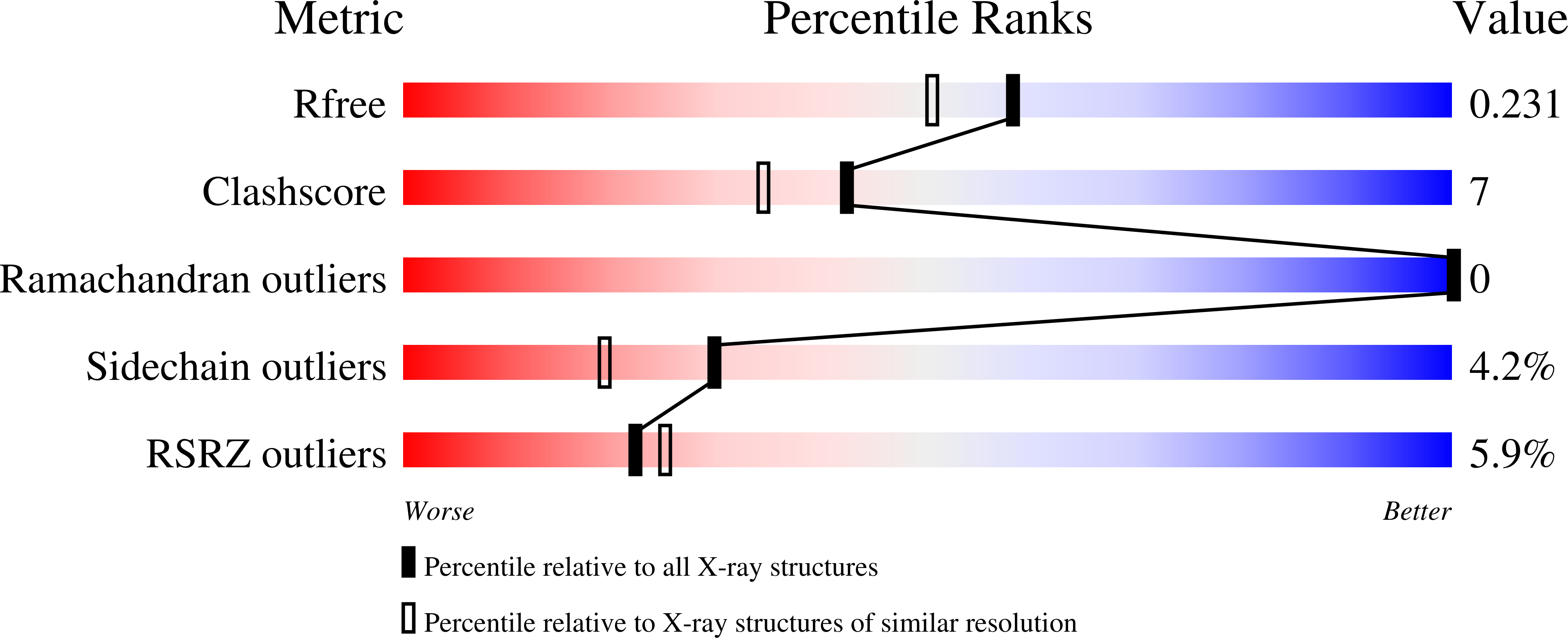
Resolution:
1.92 Å
R-Value Free:
0.23
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
C 1 2 1