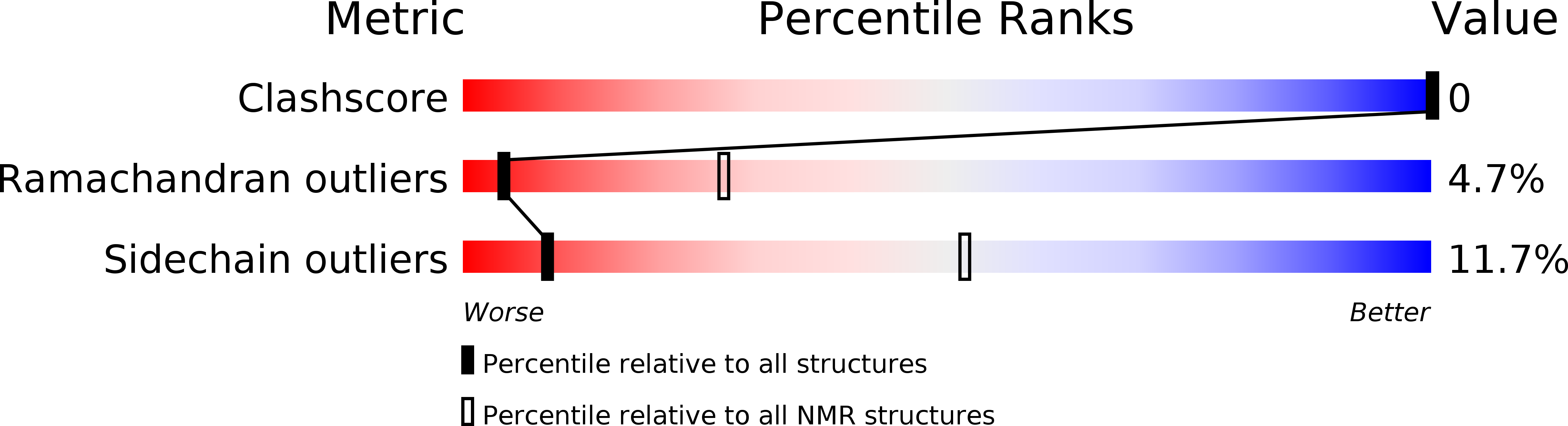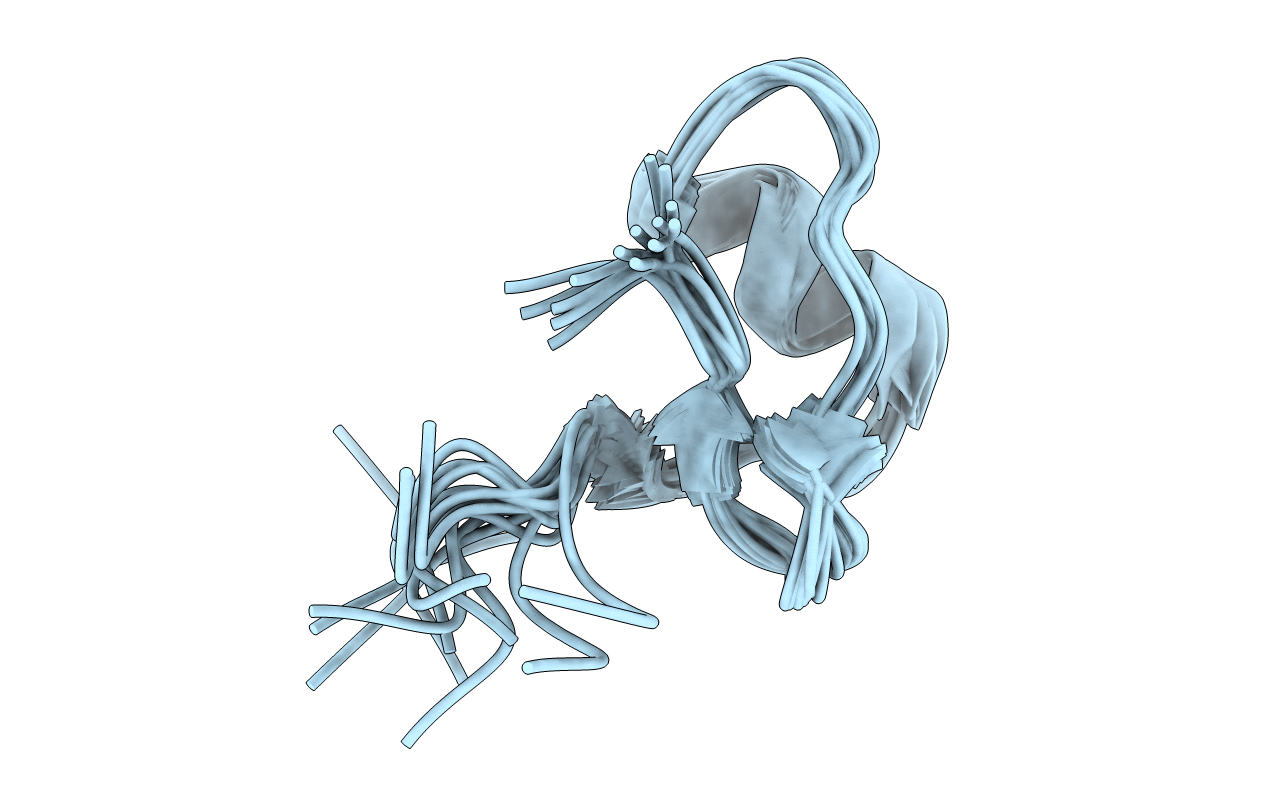
Deposition Date
2003-06-29
Release Date
2004-05-18
Last Version Date
2024-11-13
Entry Detail
PDB ID:
1PVZ
Keywords:
Title:
Solution Structure of BmP07, A Novel Potassium Channel Blocker from Scorpion Buthus martensi Karsch, 15 structures
Biological Source:
Source Organism(s):
Mesobuthus martensii (Taxon ID: 34649)
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
15
Selection Criteria:
structures with the lowest energy