Deposition Date
2003-06-21
Release Date
2003-12-16
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1PSB
Keywords:
Title:
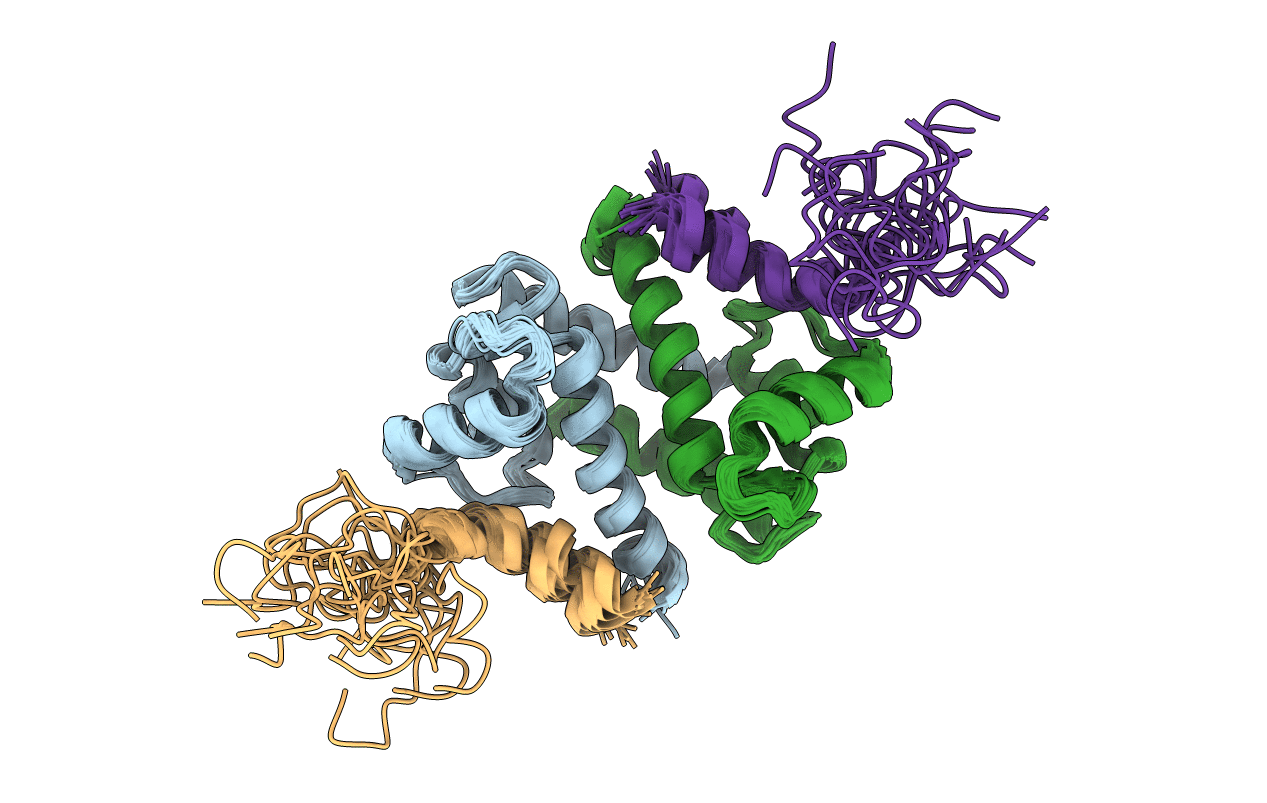
Solution structure of calcium loaded S100B complexed to a peptide from N-Terminal regulatory domain of NDR kinase.
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Expression System(s):
Method Details:
Experimental Method:
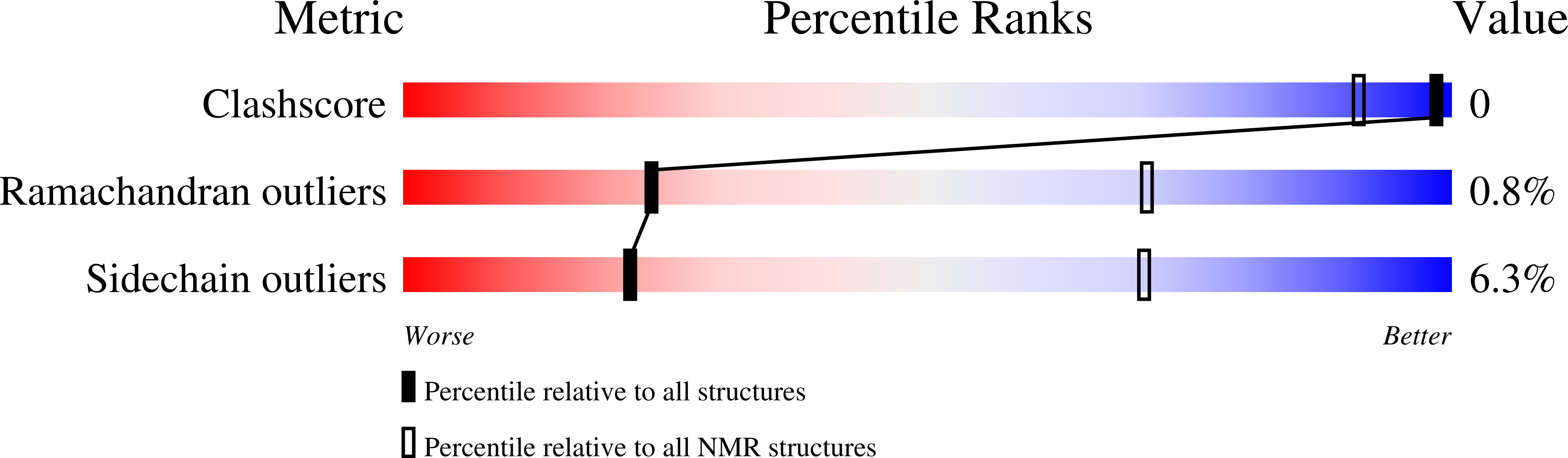
Conformers Calculated:
128
Conformers Submitted:
20
Selection Criteria:
structures with the least restraint violations,structures with the lowest energy