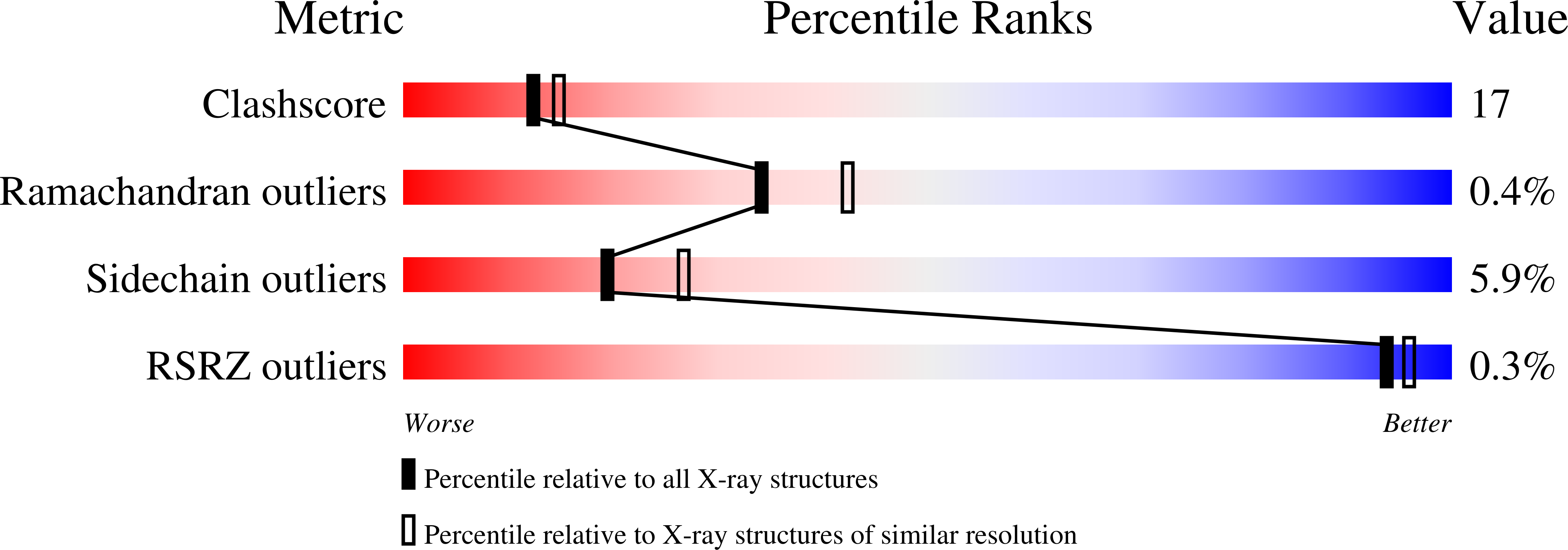Deposition Date
1988-02-04
Release Date
1989-01-09
Last Version Date
2024-10-16
Entry Detail
PDB ID:
1PRC
Keywords:
Title:
CRYSTALLOGRAPHIC REFINEMENT AT 2.3 ANGSTROMS RESOLUTION AND REFINED MODEL OF THE PHOTOSYNTHETIC REACTION CENTER FROM RHODOPSEUDOMONAS VIRIDIS
Biological Source:
Source Organism(s):
Blastochloris viridis (Taxon ID: 1079)
Method Details:
Experimental Method:
Resolution:
2.30 Å
R-Value Observed:
0.19
Space Group:
P 43 21 2