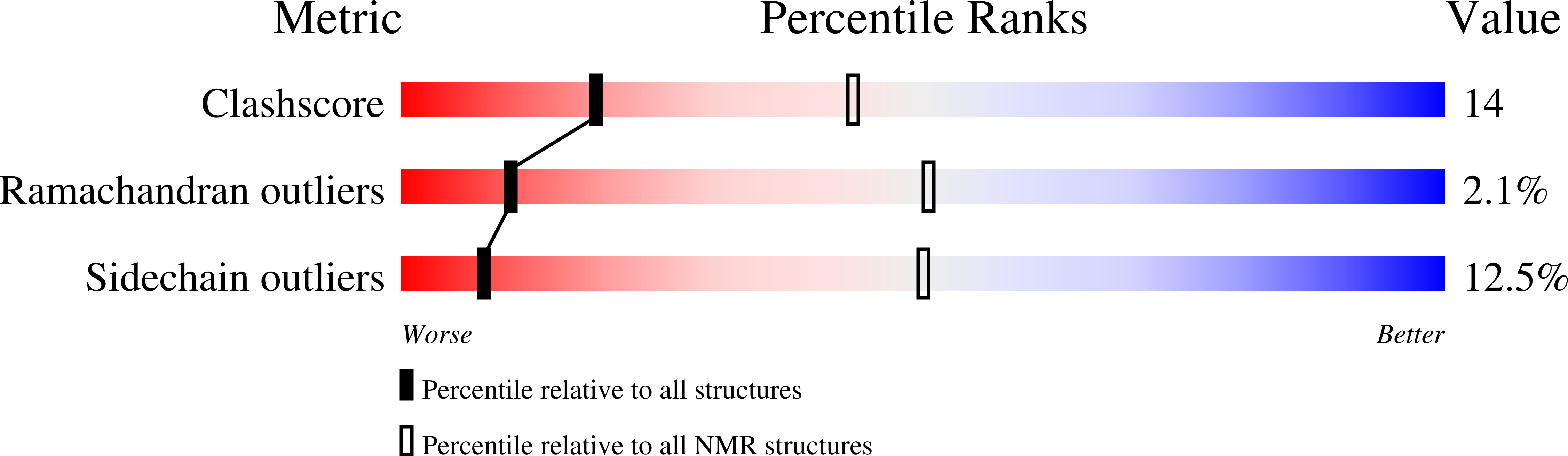
Deposition Date
2003-06-09
Release Date
2004-03-16
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1PLX
Keywords:
Title:
NMR structure of Methionine-Enkephalin in fast tumbling Bicelles/DMPG
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
80
Selection Criteria:
structures with the lowest energy