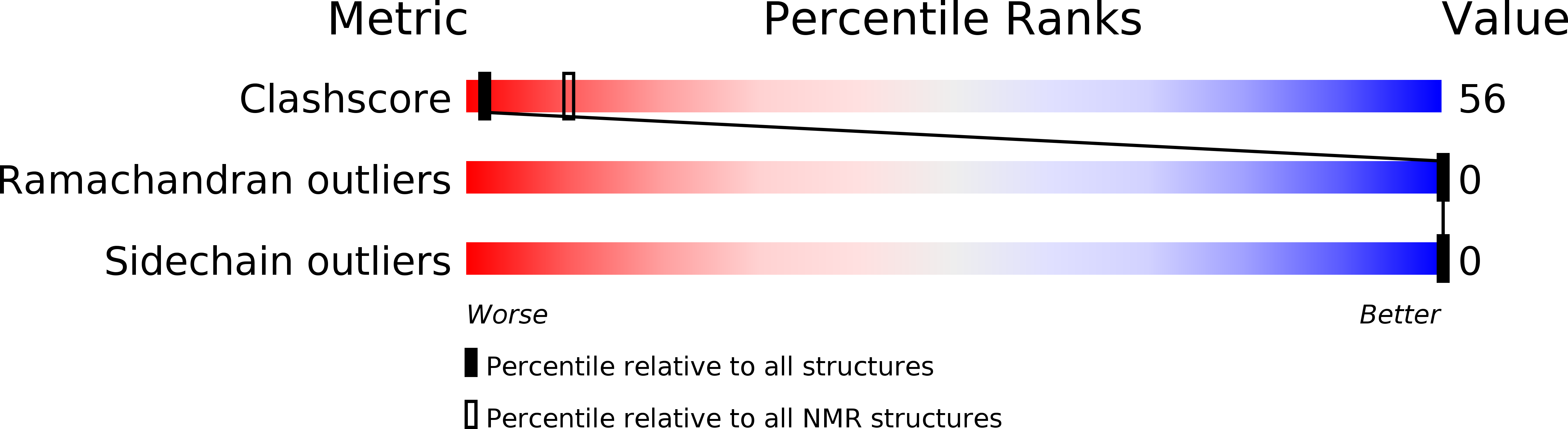Deposition Date
2003-06-02
Release Date
2004-08-10
Last Version Date
2024-05-01
Entry Detail
PDB ID:
1PJF
Keywords:
Title:
Solid State NMR structure of the Pf1 Major Coat Protein in Magnetically Aligned Bacteriophage
Biological Source:
Source Organism(s):
Pseudomonas phage Pf1 (Taxon ID: 10871)
Method Details: