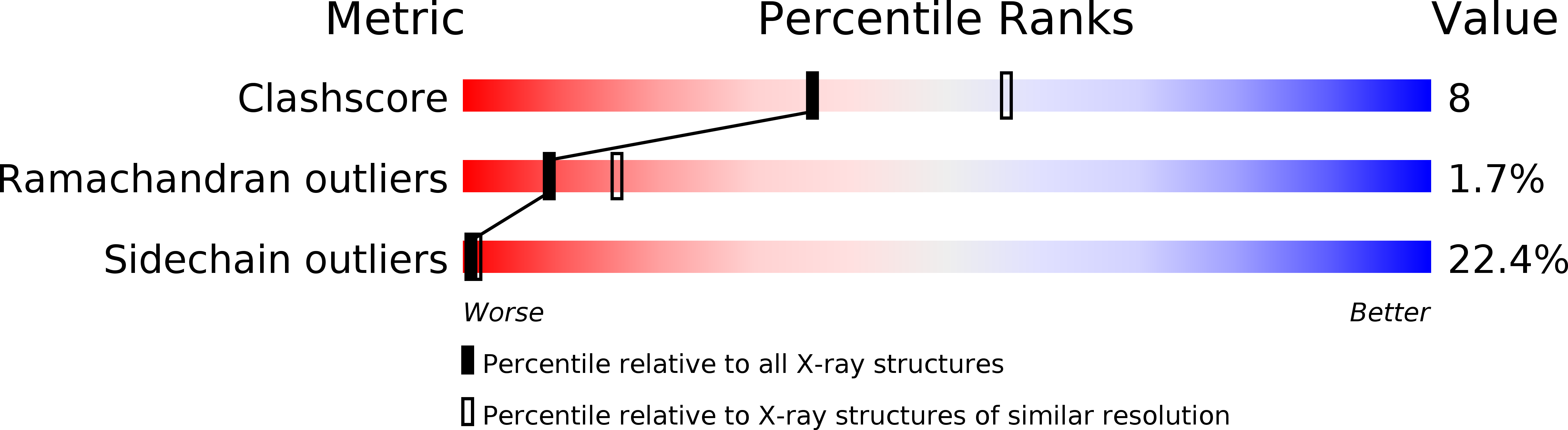
Deposition Date
1991-03-26
Release Date
1992-04-15
Last Version Date
2024-10-09
Entry Detail
PDB ID:
1PI2
Keywords:
Title:
REACTIVE SITES OF AN ANTICARCINOGENIC BOWMAN-BIRK PROTEINASE INHIBITOR ARE SIMILAR TO OTHER TRYPSIN INHIBITORS
Biological Source:
Source Organism(s):
Glycine max (Taxon ID: 3847)
Method Details:
Experimental Method:
Resolution:
2.50 Å
R-Value Observed:
0.23
Space Group:
P 41 3 2