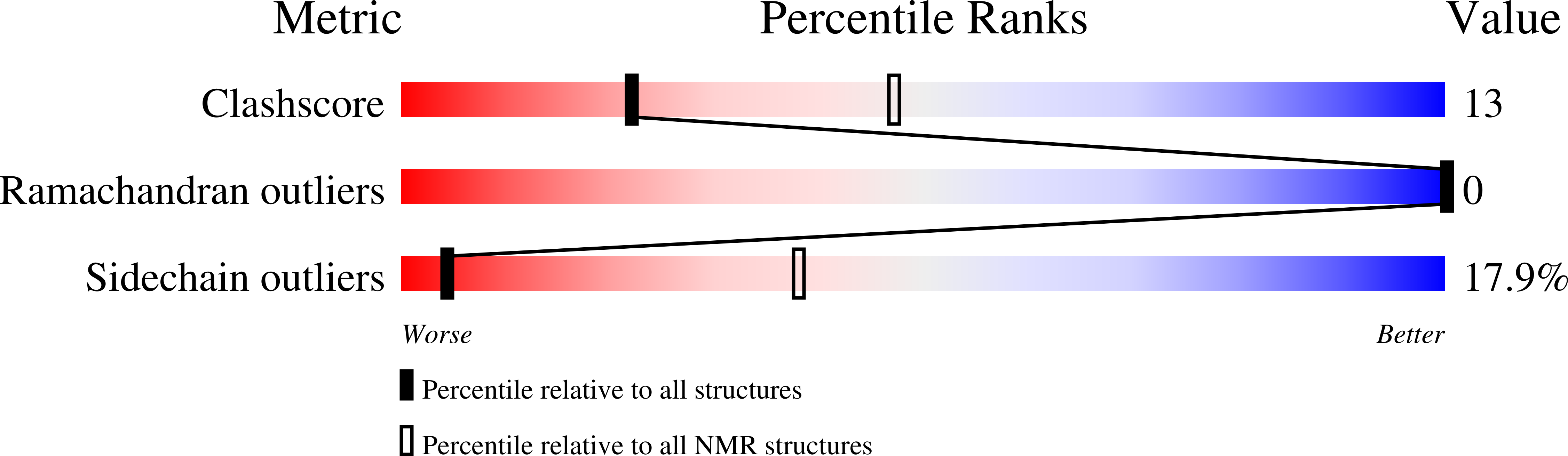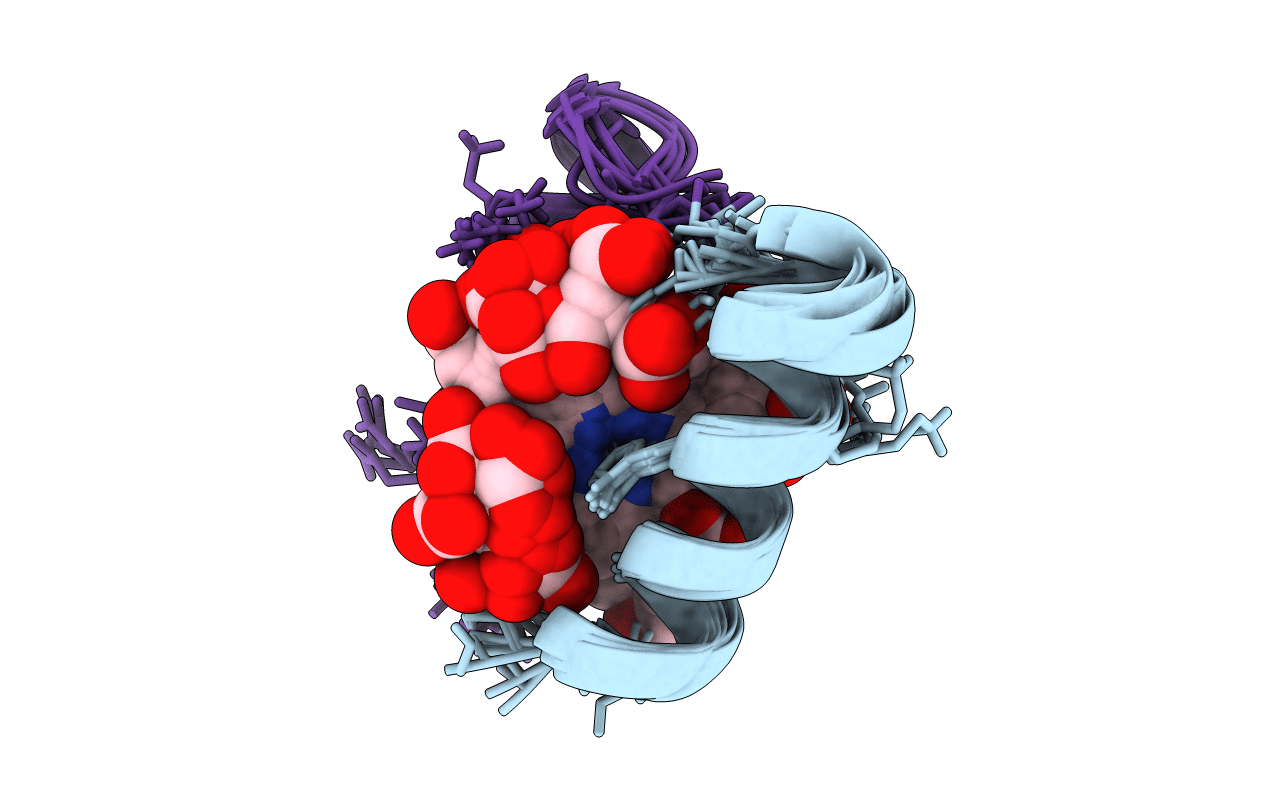
Deposition Date
2003-05-15
Release Date
2003-12-09
Last Version Date
2024-11-06
Entry Detail
PDB ID:
1PBZ
Keywords:
Title:
DE NOVO DESIGNED PEPTIDE-METALLOPORPHYRIN COMPLEX, SOLUTION STRUCTURE
Method Details:
Experimental Method:
Conformers Calculated:
40
Conformers Submitted:
14
Selection Criteria:
NO RESTRAINT VIOLATIONS GREATER THAN 0.3 ANGSTROMS