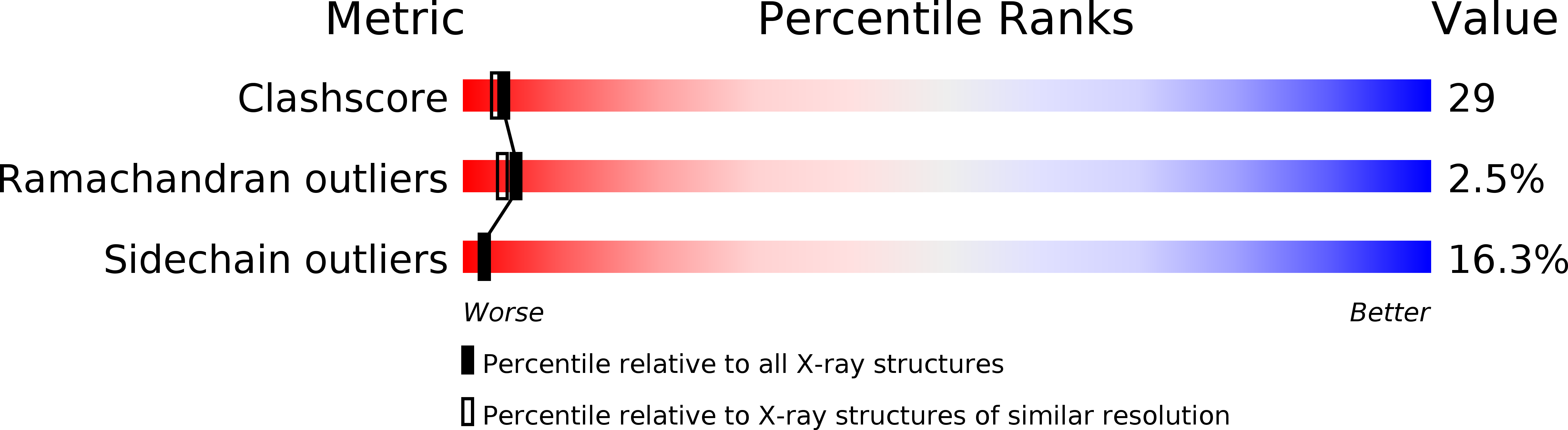
Deposition Date
1992-10-05
Release Date
1994-01-31
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1OVB
Keywords:
Title:
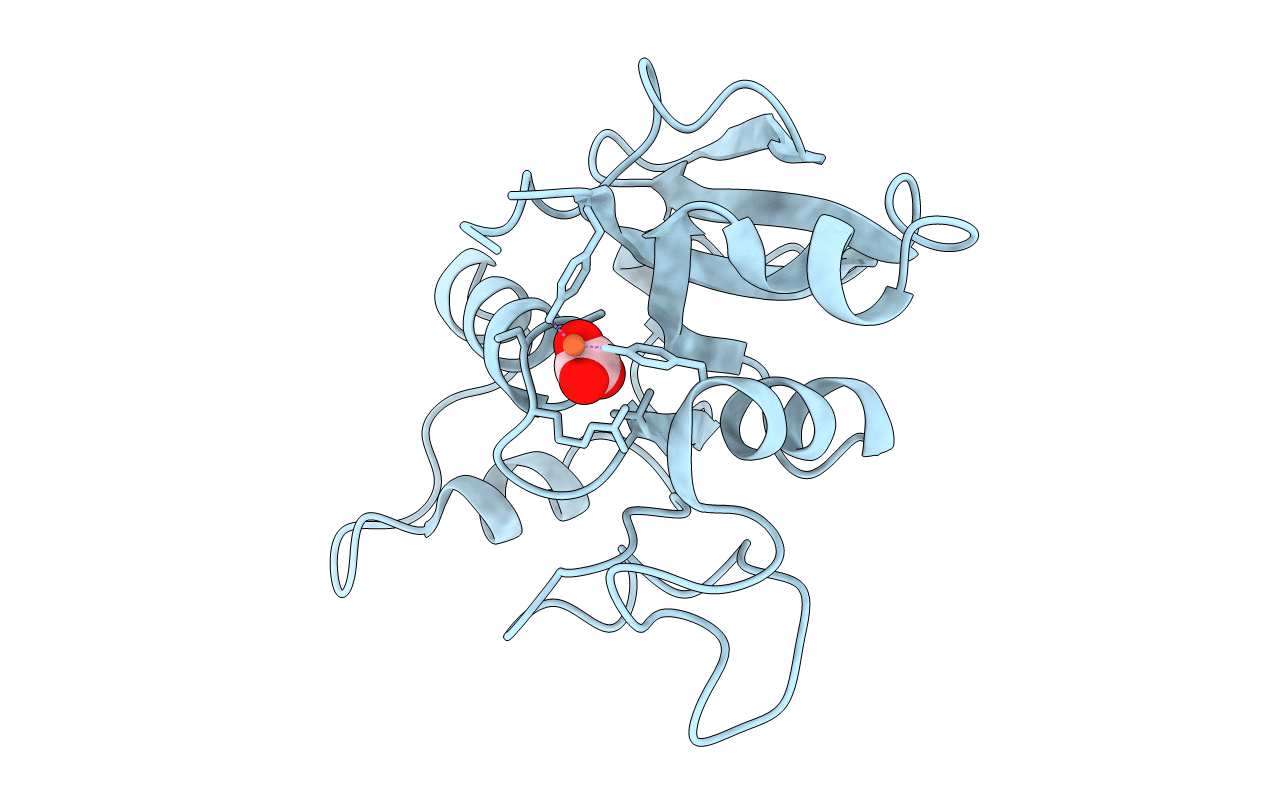
THE MECHANISM OF IRON UPTAKE BY TRANSFERRINS: THE STRUCTURE OF AN 18KD NII-DOMAIN FRAGMENT AT 2.3 ANGSTROMS RESOLUTION
Biological Source:
Method Details:
Experimental Method:
Resolution:
2.30 Å
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 31