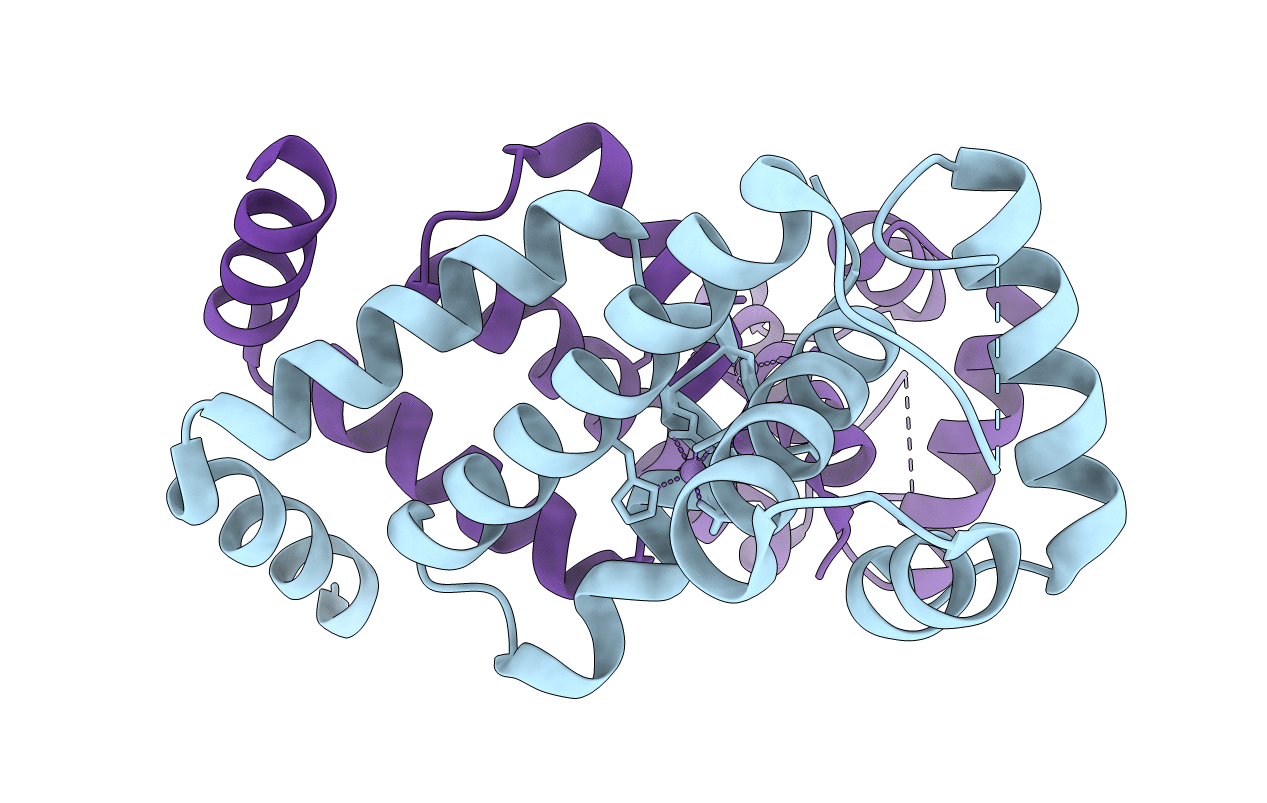
Deposition Date
2003-02-26
Release Date
2003-07-15
Last Version Date
2023-08-16
Entry Detail
PDB ID:
1ON1
Keywords:
Title:
Bacillus Subtilis Manganese Transport Regulator (Mntr) Bound To Manganese, AB Conformation.
Biological Source:
Source Organism:
Bacillus subtilis (Taxon ID: 1423)
Host Organism:
Method Details:
Experimental Method:
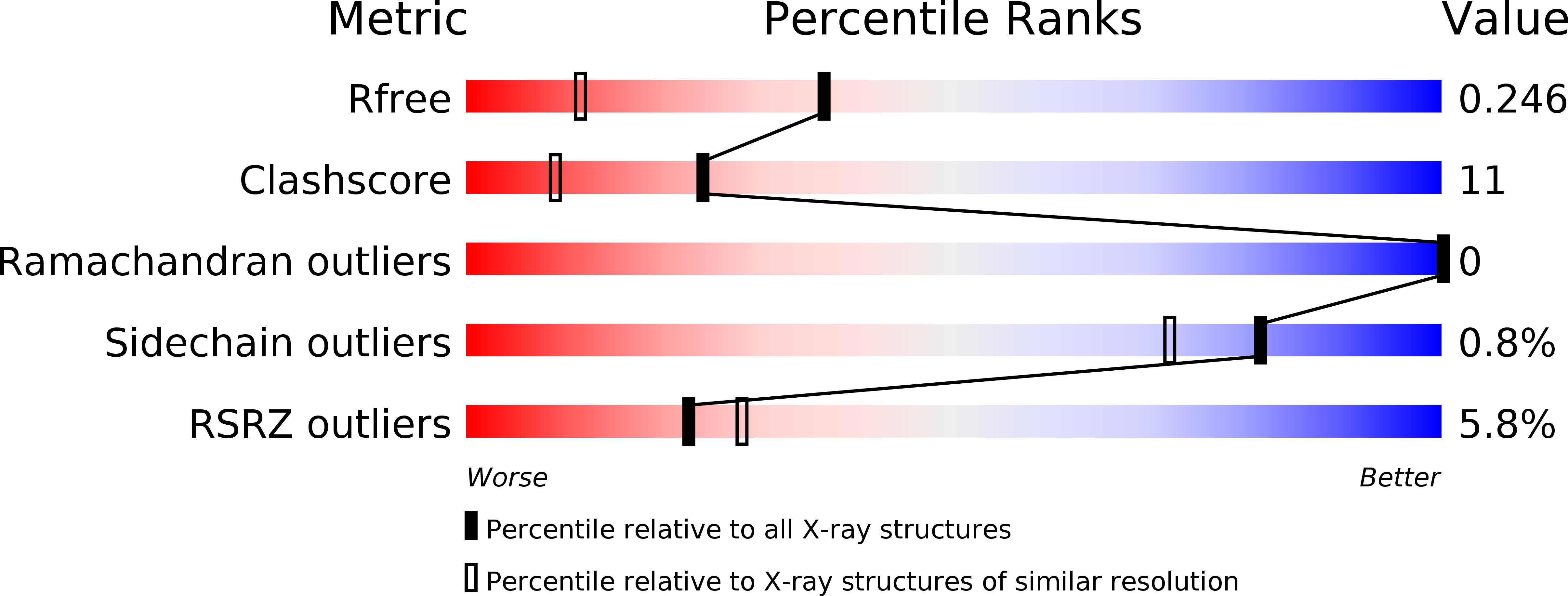
Resolution:
1.75 Å
R-Value Free:
0.24
R-Value Work:
0.21
Space Group:
P 1 21 1