Deposition Date
2003-07-03
Release Date
2004-07-08
Last Version Date
2024-05-08
Entry Detail
PDB ID:
1OJ7
Keywords:
Title:
STRUCTURAL GENOMICS, UNKNOWN FUNCTION CRYSTAL STRUCTURE OF E. COLI K-12 YQHD
Biological Source:
Source Organism:
ESCHERICHIA COLI (Taxon ID: 83333)
Host Organism:
Method Details:
Experimental Method:
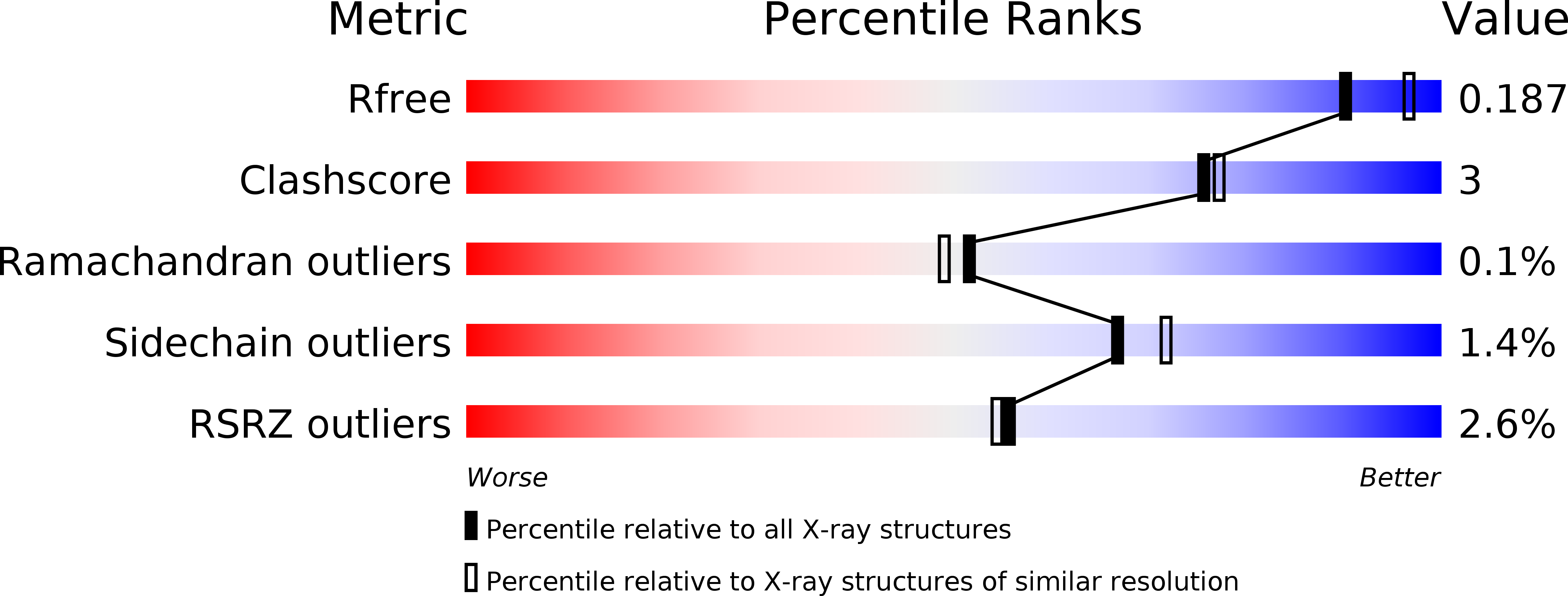
Resolution:
2.00 Å
R-Value Free:
0.16
R-Value Work:
0.14
R-Value Observed:
0.14
Space Group:
P 62