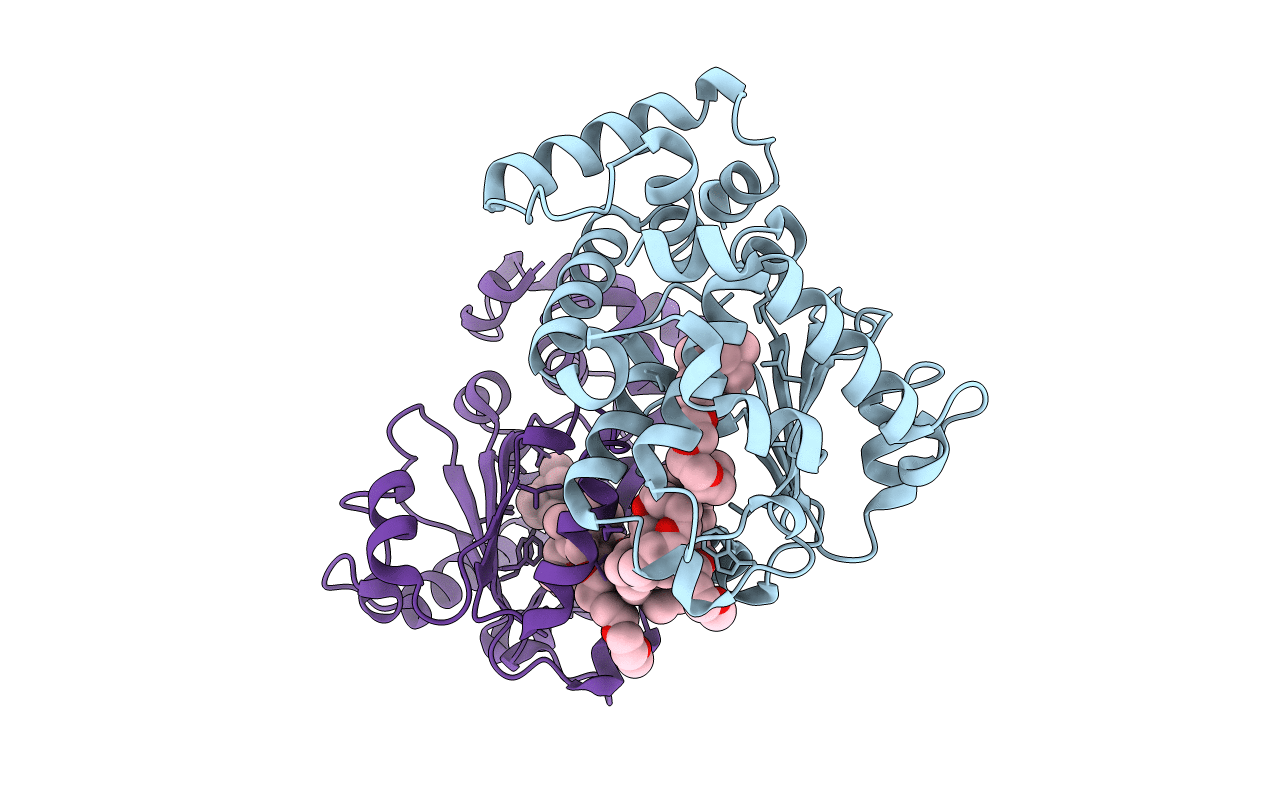
Deposition Date
2003-06-27
Release Date
2004-01-14
Last Version Date
2025-10-01
Entry Detail
PDB ID:
1OIZ
Keywords:
Title:
The Molecular Basis of Vitamin E Retention: Structure of Human Alpha-Tocopherol Transfer Protein
Biological Source:
Source Organism:
HOMO SAPIENS (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
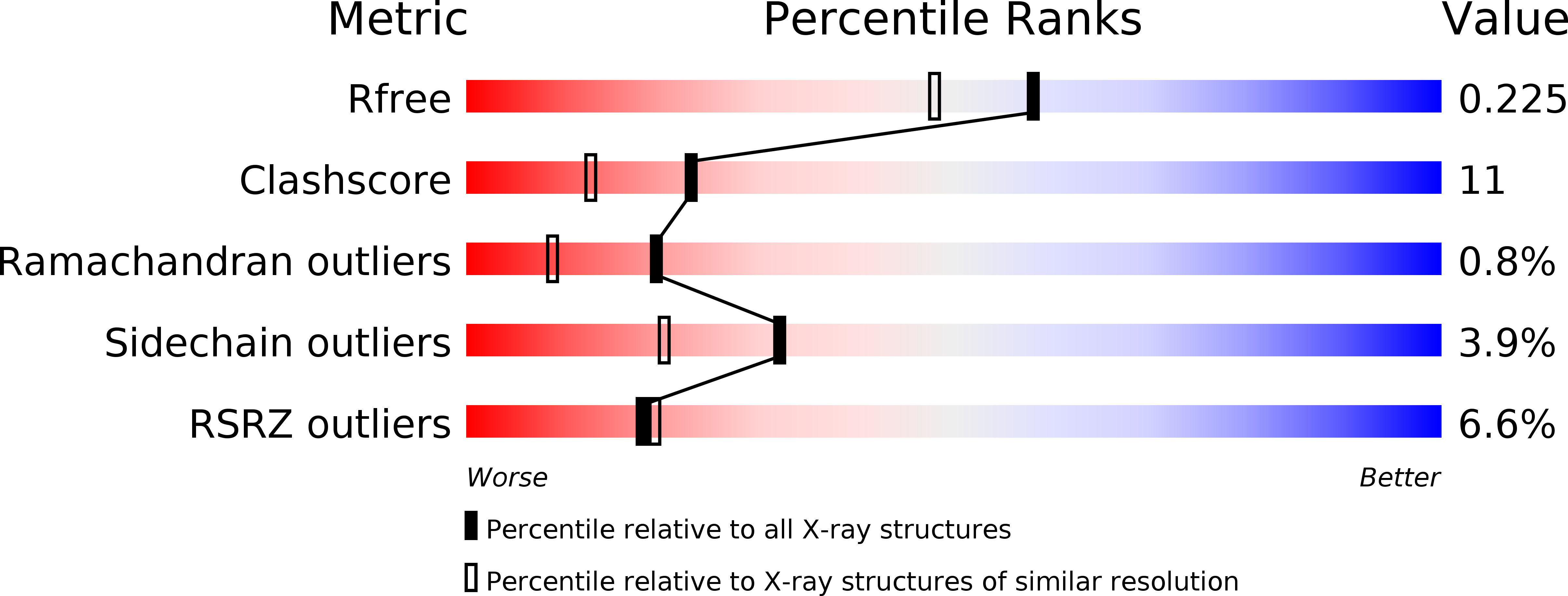
Resolution:
1.88 Å
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 1 21 1