Abstact
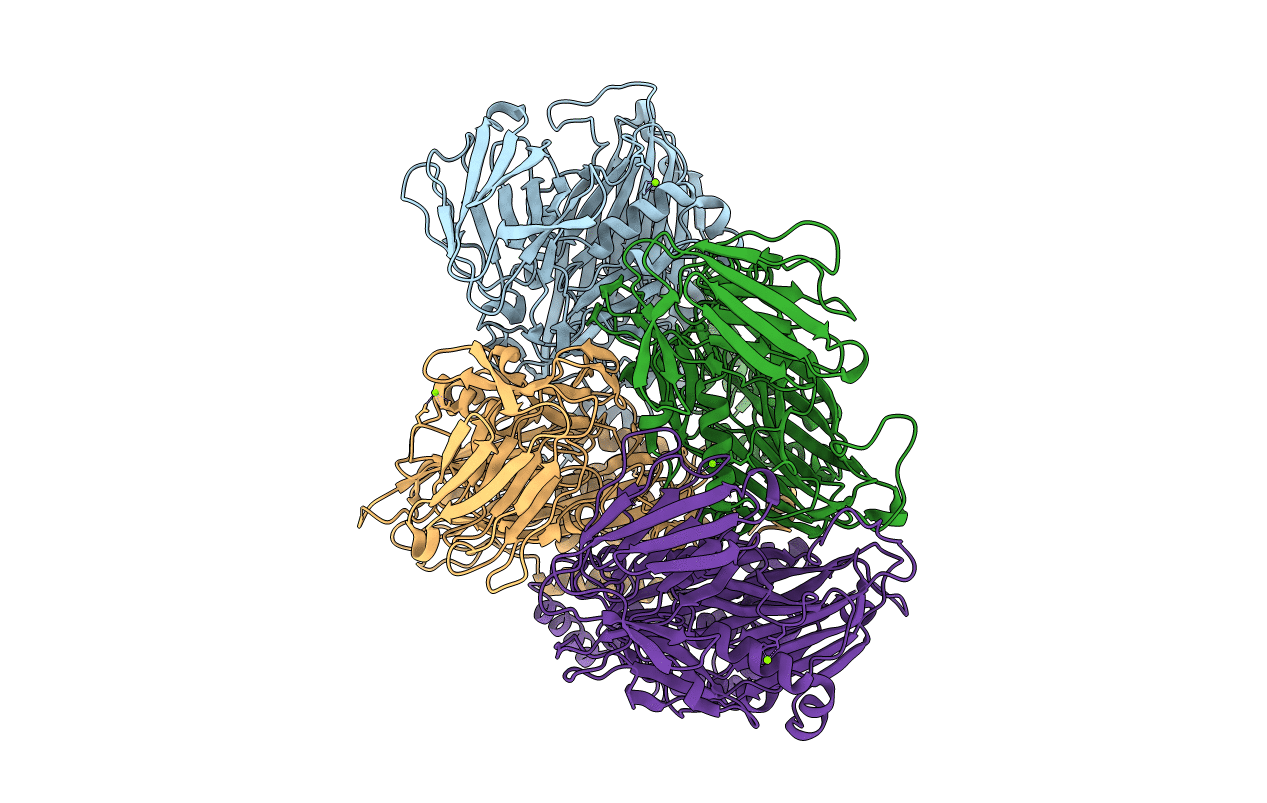
Large-scale reorganization of protein interactions characterizes many biological processes, yet few systems are accessible to biophysical studies that display this property. The capsid protein of Nudaurelia capensis omega Virus (NomegaV) has previously been characterized in two dramatically different T = 4 quasi-equivalent assembly states when expressed as virus-like particles (VLPs) in a baculovirus system. The procapsid (pH 7), is round, porous, and approximately 450 A in diameter. It converts, in vitro, to the capsid form at pH 5 and the capsid is sealed shut, shaped like an icosahedron, has a maximum diameter of 410 A and undergoes an autocatalytic cleavage at residue 570. Residues 571-644, the gamma peptide, remain associated with the particle and are partially ordered. The interconversion of these states has been previously studied by solution X-ray scattering, electron cryo microscopy (CryoEM), and site-directed mutagenesis. The particle structures appear equivalent in authentic virions and the low pH form of the expressed and assembled protein. Previously, and before the discovery of the multiple morphological forms of the VLPs, we reported the X-ray structure of authentic NomegaV at 2.8 A resolution. These coordinates defined the fold of the protein but were not refined at the time because of technical issues associated with the approximately 2.5 million reflection data set. We now report the refined, authentic virus structure that has added 29 residues to the original model and allows the description of the chemistry of molecular switching for T = 4 capsid formation and the multiple morphological forms. The amino and carboxy termini are internal, predominantly helical, and disordered to different degrees in the four structurally independent subunits; however, the refined structure shows significantly more ordered residues in this region, particularly at the amino end of the B subunit that is now seen to invade space occupied by the A subunits. These additional residues revealed a previously unnoticed strong interaction between the pentameric, gamma peptide helices of the A and B subunits that are largely proximal to the quasi-6-fold axes. One C-terminal helix is ordered in the C and D subunits and stabilizes a flat interaction in two interfaces between the protein monomers while the other, quasi-equivalent, interactions are bent. As this helix is arginine rich, the comparable, disordered region in the A and B subunits probably interacts with RNA. One of the subunit-subunit interfaces has an unusual arrangement of carboxylate side chains. Based on this observation, we propose a mechanism for the control of the pH-dependent transitions of the virus particle.