Deposition Date
2003-04-08
Release Date
2004-04-08
Last Version Date
2023-12-13
Entry Detail
PDB ID:
1OF8
Keywords:
Title:
double complex of the tyrosine sensitive DAHP Synthase from S. cerevisiae with Co2+, PEP and the E4P analogoue G3P
Biological Source:
Source Organism(s):
SACCHAROMYCES CEREVISIAE (Taxon ID: 4932)
Expression System(s):
Method Details:
Experimental Method:
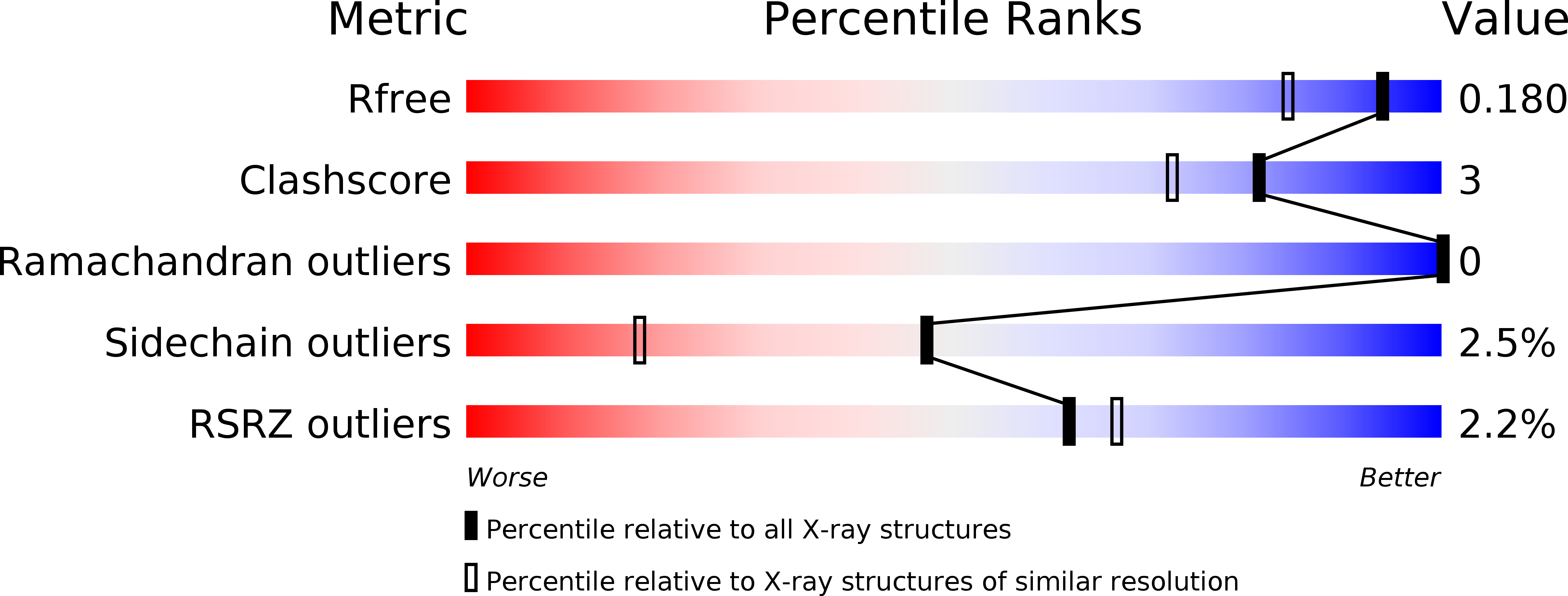
Resolution:
1.50 Å
R-Value Free:
0.16
R-Value Work:
0.12
R-Value Observed:
0.12
Space Group:
C 1 2 1